Stage-dependent alterations of progenitor cell proliferation and neurogenesis in an animal model of Wernicke-Korsakoff syndrome
- PMID: 21440532
- PMCID: PMC3087287
- DOI: 10.1016/j.brainres.2011.03.048
Stage-dependent alterations of progenitor cell proliferation and neurogenesis in an animal model of Wernicke-Korsakoff syndrome
Abstract
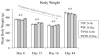
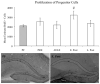
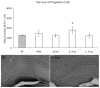
Alcohol-induced Wernicke-Korsakoff syndrome (WKS) culminates in bilateral diencephalic lesion and severe amnesia. Using the pyrithiamine-induced thiamine deficiency (PTD) animal paradigm of WKS, our laboratory has demonstrated hippocampal dysfunction in the absence of gross anatomical pathology. Extensive literature has revealed reduced hippocampal neurogenesis following a neuropathological insult, which might contribute to hippocampus-based learning and memory impairments. Thus, the current investigation was conducted to determine whether PTD treatment altered hippocampal neurogenesis in a stage-dependent fashion. Male Sprague-Dawley rats were assigned to one of 4 stages of thiamine deficiency based on behavioral symptoms: pre-symptomatic stage, ataxic stage, early post-opisthotonus stage, or the late post-opisthotonus stage. The S-phase mitotic marker 5'-bromo-2'-deoxyuridine (BrdU) was administered at the conclusion of each stage following thiamine restoration and subjects were perfused 24 hours or 28 days after BrdU to assess cellular proliferation or neurogenesis and survival, respectively. Dorsal hippocampal sections were immunostained for BrdU (proliferating cell marker), NeuN (neurons), GFAP (astrocytes), Iba-1 (microglia), and O4 (oligodendrocytes). The PTD treatment increased progenitor cell proliferation and survival during the early post-opisthotonus stage. However, levels of neurogenesis were reduced during this stage as well as the late post-opisthotonus stage where there was also an increase in astrocytogenesis. The diminished numbers of newly generated neurons (BrdU/NeuN co-localization) was paralleled by increased BrdU cells that did not co-localize with any of the phenotypic markers during these later stages. These data demonstrate that long-term alterations in neurogenesis and gliogenesis might contribute to the observed hippocampal dysfunction in the PTD model and human WKS.
Published by Elsevier B.V.
Figures

Similar articles
-
Age-related vulnerability to diencephalic amnesia produced by thiamine deficiency: the role of time of insult.Behav Brain Res. 2004 Jan 5;148(1-2):93-105. doi: 10.1016/s0166-4328(03)00208-0. Behav Brain Res. 2004. PMID: 14684251
-
Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat.Restor Neurol Neurosci. 2007;25(1):65-76. Restor Neurol Neurosci. 2007. PMID: 17473396
-
Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent.Behav Brain Res. 2001 Mar 15;119(2):167-77. doi: 10.1016/s0166-4328(00)00350-8. Behav Brain Res. 2001. PMID: 11165332
-
Thiamine deficiency induced neurochemical, neuroanatomical, and neuropsychological alterations: a reappraisal.ScientificWorldJournal. 2013 Oct 21;2013:309143. doi: 10.1155/2013/309143. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24235882 Free PMC article. Review.
-
Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment.Neurobiol Learn Mem. 2011 Nov;96(4):596-608. doi: 10.1016/j.nlm.2011.01.003. Epub 2011 Jan 21. Neurobiol Learn Mem. 2011. PMID: 21256970 Free PMC article. Review.
Cited by
-
Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery.Neuropsychol Rev. 2012 Jun;22(2):195-209. doi: 10.1007/s11065-012-9194-1. Epub 2012 Apr 13. Neuropsychol Rev. 2012. PMID: 22528861 Free PMC article. Review.
-
Stem Cell Therapy and Thiamine Deficiency-Induced Brain Damage.Neurochem Res. 2024 Jun;49(6):1450-1467. doi: 10.1007/s11064-024-04137-5. Epub 2024 May 9. Neurochem Res. 2024. PMID: 38720090 Review.
-
Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations.Neurochem Res. 2015 Feb;40(2):353-61. doi: 10.1007/s11064-014-1430-z. Epub 2014 Oct 9. Neurochem Res. 2015. PMID: 25297573 Review.
-
Time to put the mammillothalamic pathway into context.Neurosci Biobehav Rev. 2021 Feb;121:60-74. doi: 10.1016/j.neubiorev.2020.11.031. Epub 2020 Dec 9. Neurosci Biobehav Rev. 2021. PMID: 33309908 Free PMC article. Review.
-
Thiamine deficiency degrades the link between spatial behavior and hippocampal synapsin I and phosphorylated synapsin I protein levels.Behav Brain Res. 2012 Jul 1;232(2):421-5. doi: 10.1016/j.bbr.2012.04.004. Epub 2012 Apr 9. Behav Brain Res. 2012. PMID: 22507301 Free PMC article.
References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol. Rev. 2005;85:523–569. - PubMed
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of tie in new memories. Nat. Neurosci. 2006;9:723–727. - PubMed
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 2000;296:21–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

