Glucokinase inhibitor glucosamine stimulates feeding and activates hypothalamic neuropeptide Y and orexin neurons
- PMID: 21440571
- PMCID: PMC3133639
- DOI: 10.1016/j.bbr.2011.03.043
Glucokinase inhibitor glucosamine stimulates feeding and activates hypothalamic neuropeptide Y and orexin neurons
Abstract
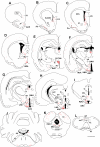
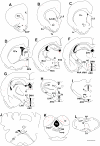
Maintaining glucose levels within the appropriate physiological range is necessary for survival. The identification of specific neuronal populations, within discreet brain regions, sensitive to changes in glucose concentration has led to the hypothesis of a central glucose-sensing system capable of directly modulating feeding behaviour. Glucokinase (GK) has been identified as a glucose-sensor responsible for detecting such changes both within the brain and the periphery. We previously reported that antagonism of centrally expressed GK by administration of glucosamine (GSN) was sufficient to induce protective glucoprivic feeding in rats. Here we examine a neurochemical mechanism underlying this effect and report that GSN stimulated food intake is highly correlated with the induction of the neuronal activation marker cFOS within two nuclei with a demonstrated role in central glucose sensing and appetite, the arcuate nucleus of the hypothalamus (ARC) and lateral hypothalamic area (LHA). Furthermore, GSN stimulated cFOS within the ARC was observed in orexigenic neurons expressing the endogenous melanocortin receptor antagonist agouti-related peptide (AgRP) and neuropeptide Y (NPY), but not those expressing the anorectic endogenous melanocortin receptor agonist alpha-melanocyte stimulating hormone (α-MSH). In the LHA, GSN stimulated cFOS was found within arousal and feeding associated orexin/hypocretin (ORX), but not orexigenic melanin-concentrating hormone (MCH) expressing neurons. Our data suggest that GK within these specific feeding and arousal related populations of AgRP/NPY and ORX neurons may play a modulatory role in the sensing of and appetitive response to hypoglycaemia.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
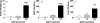
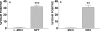
Similar articles
-
Neurochemical phenotype of hypothalamic neurons showing Fos expression 23 h after intracranial AgRP.Am J Physiol Regul Integr Comp Physiol. 2002 Jun;282(6):R1773-81. doi: 10.1152/ajpregu.00019.2002. Am J Physiol Regul Integr Comp Physiol. 2002. PMID: 12010760
-
Interactions of neuropeptide Y, hypocretin-I (orexin A) and melanin-concentrating hormone on feeding in rats.Brain Res. 2002 Jul 19;944(1-2):232-8. doi: 10.1016/s0006-8993(02)02941-4. Brain Res. 2002. PMID: 12106685
-
Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus.Neuroreport. 2000 Jan 17;11(1):117-21. doi: 10.1097/00001756-200001170-00023. Neuroreport. 2000. PMID: 10683841
-
The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box.Proc Nutr Soc. 2000 Aug;59(3):385-96. doi: 10.1017/s0029665100000434. Proc Nutr Soc. 2000. PMID: 10997654 Review.
-
Neuropeptides controlling energy balance: orexins and neuromedins.Handb Exp Pharmacol. 2012;(209):77-109. doi: 10.1007/978-3-642-24716-3_4. Handb Exp Pharmacol. 2012. PMID: 22249811 Free PMC article. Review.
Cited by
-
The Ciji-Hua'ai-Baosheng II Formula Attenuates Chemotherapy-Induced Anorexia in Mice With H22 Hepatocellular Carcinoma.Front Pharmacol. 2021 Aug 19;12:715824. doi: 10.3389/fphar.2021.715824. eCollection 2021. Front Pharmacol. 2021. PMID: 34489705 Free PMC article.
-
The identification of neuropeptide Y receptor subtype involved in phenylpropanolamine-induced increase in oxidative stress and appetite suppression.Neuromolecular Med. 2013 Mar;15(1):159-68. doi: 10.1007/s12017-012-8206-x. Epub 2012 Nov 20. Neuromolecular Med. 2013. PMID: 23179670
-
Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia.Endocr Rev. 2019 Jun 1;40(3):768-788. doi: 10.1210/er.2018-00226. Endocr Rev. 2019. PMID: 30689785 Free PMC article. Review.
-
Minireview: The value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit.Endocrinology. 2011 Nov;152(11):4019-32. doi: 10.1210/en.2010-1458. Epub 2011 Aug 30. Endocrinology. 2011. PMID: 21878511 Free PMC article. Review.
-
Stimulation of feeding by three different glucose-sensing mechanisms requires hindbrain catecholamine neurons.Am J Physiol Regul Integr Comp Physiol. 2014 Feb 15;306(4):R257-64. doi: 10.1152/ajpregu.00451.2013. Epub 2013 Dec 31. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24381177 Free PMC article.
References
-
- Garfield A.S., Lam D.D., Marston O.J., Przydzial M.J., Heisler L.K. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20(5):203–215. - PubMed
-
- Saper C.B., Chou T.C., Elmquist J.K. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. - PubMed
-
- Fioramonti X., Contie S., Song Z., Routh V.H., Lorsignol A., Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56(5):1219–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

