SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion
- PMID: 21440942
- PMCID: PMC3129404
- DOI: 10.1016/j.ophtha.2010.11.020
SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion
Abstract
Purpose: To investigate in The Standard Care versus COrticosteroid for REtinal Vein Occlusion (SCORE) Study: (1) incidences of neovascular events and retinal capillary nonperfusion (abbreviated as "nonperfusion"), and their relationship with treatment groups; (2) neovascular incidences by nonperfusion status; and (3) pertinent baseline factors for their potential risk for neovascular events.
Design: Two multicenter, randomized clinical trials, 1 evaluating participants with central retinal vein occlusion (CRVO) and the other evaluating participants with branch retinal vein occlusion (BRVO).
Participants: At 36 months, data were available for 81 participants with CRVO and 128 with BRVO.
Intervention: Standard care (observation or grid photocoagulation) versus 1 or 4 mg intravitreal triamcinolone.
Main outcome measures: Neovascularization of the iris (NVI), neovascular glaucoma (NVG), disc or retinal neovascularization (NVD/NVE), preretinal or vitreous hemorrhage (PRH/VH), and nonperfusion.
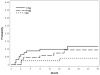
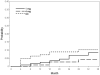
Results: The cumulative 36-month incidences for CRVO and BRVO eyes, respectively, were 8.5% and 2.4% for NVI or NVG; 8.8% and 7.6% for NVD/NVE or PRH/VH. There were no differences in incidences of neovascular events or risk of nonperfusion when comparing the 3 treatment groups within diseases. For CRVO at 36 months, 16.6% of eyes with ≥5.5 disc areas of nonperfusion versus 4.0% of eyes with <5.5 disc areas of nonperfusion developed NVG (P = 0.0003); for BRVO at 36 months, 14.6% versus 2.4% developed NVD/NVE (P<0.0001). Similar results were noted for most other neovascular events. Nonperfusion was the only significant baseline factor for neovascularization in BRVO, with the risk of a neovascular event increasing with greater disc areas of nonperfusion, and the highest risk noted at ≥5.5 disc areas.
Conclusions: In the SCORE Study, triamcinolone treatment was not associated with lower incidences of neovascular events or nonperfusion status compared with observation or grid photocoagulation. Cumulative 36-month incidences for most neovascular events were significantly higher for nonperfused than perfused eyes. Greater baseline disc areas of nonperfusion increased the risk of neovascularization in BRVO but not CRVO eyes, possibly owing to obscuration of retinal capillary details caused by dense hemorrhage at baseline for CRVO eyes. Increased risk of neovascularization was noted below the historical threshold of 10 disc areas of nonperfusion for retinal vein occlusion.
Copyright © 2011 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98:271–82. - PubMed
-
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. - PubMed
-
- Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. - PubMed
-
- Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

