Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans
- PMID: 21441208
- PMCID: PMC3122321
- DOI: 10.1534/genetics.111.128538
Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans
Abstract
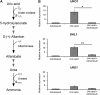
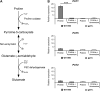
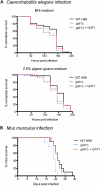
Proper regulation of metabolism is essential to maximizing fitness of organisms in their chosen environmental niche. Nitrogen metabolite repression is an example of a regulatory mechanism in fungi that enables preferential utilization of easily assimilated nitrogen sources, such as ammonium, to conserve resources. Here we provide genetic, transcriptional, and phenotypic evidence of nitrogen metabolite repression in the human pathogen Cryptococcus neoformans. In addition to loss of transcriptional activation of catabolic enzyme-encoding genes of the uric acid and proline assimilation pathways in the presence of ammonium, nitrogen metabolite repression also regulates the production of the virulence determinants capsule and melanin. Since GATA transcription factors are known to play a key role in nitrogen metabolite repression, bioinformatic analyses of the C. neoformans genome were undertaken and seven predicted GATA-type genes were identified. A screen of these deletion mutants revealed GAT1, encoding the only global transcription factor essential for utilization of a wide range of nitrogen sources, including uric acid, urea, and creatinine-three predominant nitrogen constituents found in the C. neoformans ecological niche. In addition to its evolutionarily conserved role in mediating nitrogen metabolite repression and controlling the expression of catabolic enzyme and permease-encoding genes, Gat1 also negatively regulates virulence traits, including infectious basidiospore production, melanin formation, and growth at high body temperature (39°-40°). Conversely, Gat1 positively regulates capsule production. A murine inhalation model of cryptococcosis revealed that the gat1Δ mutant is slightly more virulent than wild type, indicating that Gat1 plays a complex regulatory role during infection.
Figures


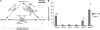


Similar articles
-
Characterization of an Nmr homolog that modulates GATA factor-mediated nitrogen metabolite repression in Cryptococcus neoformans.PLoS One. 2012;7(3):e32585. doi: 10.1371/journal.pone.0032585. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470421 Free PMC article.
-
The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans.Fungal Genet Biol. 2011 Feb;48(2):192-9. doi: 10.1016/j.fgb.2010.07.011. Epub 2010 Jul 29. Fungal Genet Biol. 2011. PMID: 20673806
-
Differences in nitrogen metabolism between Cryptococcus neoformans and C. gattii, the two etiologic agents of cryptococcosis.PLoS One. 2012;7(3):e34258. doi: 10.1371/journal.pone.0034258. Epub 2012 Mar 27. PLoS One. 2012. PMID: 22479580 Free PMC article.
-
Cryptococcus neoformans: virulence and host defences.Med Mycol. 1998;36 Suppl 1:79-86. Med Mycol. 1998. PMID: 9988495 Review.
-
Cryptococcus neoformans: a sugar-coated killer with designer genes.FEMS Immunol Med Microbiol. 2005 Sep 1;45(3):395-404. doi: 10.1016/j.femsim.2005.06.005. FEMS Immunol Med Microbiol. 2005. PMID: 16055314 Review.
Cited by
-
Characterization of an Nmr homolog that modulates GATA factor-mediated nitrogen metabolite repression in Cryptococcus neoformans.PLoS One. 2012;7(3):e32585. doi: 10.1371/journal.pone.0032585. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470421 Free PMC article.
-
Characterization of the complete uric acid degradation pathway in the fungal pathogen Cryptococcus neoformans.PLoS One. 2013 May 7;8(5):e64292. doi: 10.1371/journal.pone.0064292. Print 2013. PLoS One. 2013. PMID: 23667704 Free PMC article.
-
A Limited Number of Amino Acid Permeases Are Crucial for Cryptococcus neoformans Survival and Virulence.Int J Microbiol. 2024 Aug 8;2024:5566438. doi: 10.1155/2024/5566438. eCollection 2024. Int J Microbiol. 2024. PMID: 39148675 Free PMC article. Review.
-
A Transcriptional Regulatory Map of Iron Homeostasis Reveals a New Control Circuit for Capsule Formation in Cryptococcus neoformans.Genetics. 2020 Aug;215(4):1171-1189. doi: 10.1534/genetics.120.303270. Epub 2020 Jun 24. Genetics. 2020. PMID: 32580959 Free PMC article.
-
Pleiotropic roles of LAMMER kinase, Lkh1 in stress responses and virulence of Cryptococcus neoformans.Front Cell Infect Microbiol. 2024 May 7;14:1369301. doi: 10.3389/fcimb.2024.1369301. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38774630 Free PMC article.
References
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410 - PubMed
-
- Arst H. N., Jr, Cove D. J., 1973. Nitrogen metabolite repression in Aspergillus nidulans. Mol. Gen. Genet. 126: 111–141 - PubMed
-
- Berger H., Pachlinger R., Morozov I., Goller S., Narendja F., et al. , 2006. The GATA factor AreA regulates localization and in vivo binding site occupancy of the nitrate activator NirA. Mol. Microbiol. 59: 433–446 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

