Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation
- PMID: 21441330
- PMCID: PMC3126457
- DOI: 10.1128/AEM.00081-11
Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation
Abstract
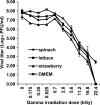
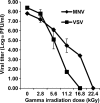
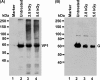
Gamma irradiation is a nonthermal processing technology that has been used for the preservation of a variety of food products. This technology has been shown to effectively inactivate bacterial pathogens. Currently, the FDA has approved doses of up to 4.0 kGy to control food-borne pathogens in fresh iceberg lettuce and spinach. However, whether this dose range effectively inactivates food-borne viruses is less understood. We have performed a systematic study on the inactivation of a human norovirus surrogate (murine norovirus 1 [MNV-1]), human norovirus virus-like particles (VLPs), and vesicular stomatitis virus (VSV) by gamma irradiation. We demonstrated that MNV-1 and human norovirus VLPs were resistant to gamma irradiation. For MNV-1, only a 1.7- to 2.4-log virus reduction in fresh produce at the dose of 5.6 kGy was observed. However, VSV was more susceptible to gamma irradiation, and a 3.3-log virus reduction at a dose of 5.6 kGy in Dulbecco's modified Eagle medium (DMEM) was achieved. We further demonstrated that gamma irradiation disrupted virion structure and degraded viral proteins and genomic RNA, which resulted in virus inactivation. Using human norovirus VLPs as a model, we provide the first evidence that the capsid of human norovirus has stability similar to that of MNV-1 after exposure to gamma irradiation. Overall, our results suggest that viruses are much more resistant to irradiation than bacterial pathogens. Although gamma irradiation used to eliminate the virus contaminants in fresh produce by the FDA-approved irradiation dose limits seems impractical, this technology may be practical to inactivate viruses for other purposes, such as sterilization of medical equipment.
Figures
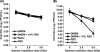
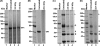



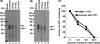
Similar articles
-
Electron beam inactivation of Tulane virus on fresh produce, and mechanism of inactivation of human norovirus surrogates by electron beam irradiation.Int J Food Microbiol. 2015 Apr 2;198:28-36. doi: 10.1016/j.ijfoodmicro.2014.12.024. Epub 2015 Jan 5. Int J Food Microbiol. 2015. PMID: 25590261
-
Electron-beam inactivation of a norovirus surrogate in fresh produce and model systems.J Food Prot. 2011 Jul;74(7):1155-60. doi: 10.4315/0362-028X.JFP-10-405. J Food Prot. 2011. PMID: 21740718
-
Inactivation efficiency and mechanism of UV-TiO2 photocatalysis against murine norovirus using a solidified agar matrix.Int J Food Microbiol. 2016 Dec 5;238:256-264. doi: 10.1016/j.ijfoodmicro.2016.09.025. Epub 2016 Sep 28. Int J Food Microbiol. 2016. PMID: 27705845
-
[Current topics on inactivation of norovirus].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2011;(129):37-54. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2011. PMID: 22259842 Review. Japanese.
-
The Influence of Simulated Organic Matter on the Inactivation of Viruses: A Review.Viruses. 2024 Jun 26;16(7):1026. doi: 10.3390/v16071026. Viruses. 2024. PMID: 39066189 Free PMC article. Review.
Cited by
-
Electromagnetic waves destabilize the SARS-CoV-2 Spike protein and reduce SARS-CoV-2 Virus-Like Particle (SC2-VLP) infectivity.bioRxiv [Preprint]. 2024 Sep 12:2024.09.11.612487. doi: 10.1101/2024.09.11.612487. bioRxiv. 2024. Update in: Sci Rep. 2025 May 15;15(1):16836. doi: 10.1038/s41598-025-01896-1. PMID: 39314332 Free PMC article. Updated. Preprint.
-
Murine Norovirus Interaction with Pseudomonas aeruginosa Biofilm in a Dynamic Bioreactor.Food Environ Virol. 2021 Dec;13(4):485-492. doi: 10.1007/s12560-021-09490-0. Epub 2021 Jul 27. Food Environ Virol. 2021. PMID: 34313942
-
The inactivation and destruction of viruses by reactive oxygen species generated through physical and cold atmospheric plasma techniques: Current status and perspectives.J Adv Res. 2023 Jan;43:59-71. doi: 10.1016/j.jare.2022.03.002. Epub 2022 Mar 9. J Adv Res. 2023. PMID: 36585115 Free PMC article. Review.
-
Spaceflight Virology: What Do We Know about Viral Threats in the Spaceflight Environment?Astrobiology. 2022 Feb;22(2):210-224. doi: 10.1089/ast.2021.0009. Epub 2022 Jan 3. Astrobiology. 2022. PMID: 34981957 Free PMC article. Review.
-
Fate of Foodborne Viruses in the "Farm to Fork" Chain of Fresh Produce.Compr Rev Food Sci Food Saf. 2015 Nov;14(6):755-770. doi: 10.1111/1541-4337.12163. Epub 2015 Oct 8. Compr Rev Food Sci Food Saf. 2015. PMID: 32313514 Free PMC article.
References
-
- Adler J. L., Zickl R. 1969. Winter vomiting disease. J. Infect. Dis. 119:668–673 - PubMed
-
- Allwood P. B., et al. 2004. Occurrence of Escherichia coli, noroviruses, and F-specific coliphages in fresh market-ready produce. J. Food Prot. 67:2387–2390 - PubMed
-
- Baert L., Debevere J., Uyttendaele M. 2009. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 131:83–94 - PubMed
-
- Baert L., Uyttendaele M., Vermeersch M., Van Coillie E., Debevere J. 2008. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J. Food Prot. 71:1590–1597 - PubMed
-
- Bari M. L., et al. 2005. Effectiveness of irradiation treatments in inactivating Listeria monocytogenes on fresh vegetables at refrigeration temperature. J. Food Prot. 68:318–323 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

