Uncovering a role for the tail of the Dictyostelium discoideum SadA protein in cell-substrate adhesion
- PMID: 21441344
- PMCID: PMC3127660
- DOI: 10.1128/EC.00221-10
Uncovering a role for the tail of the Dictyostelium discoideum SadA protein in cell-substrate adhesion
Abstract
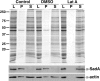
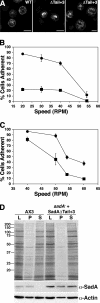
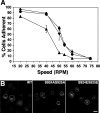
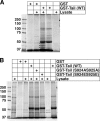
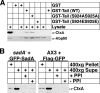
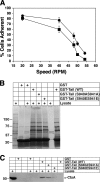
Previous work from our laboratory showed that the Dictyostelium discoideum SadA protein plays a central role in cell-substrate adhesion. SadA null cells exhibit a loss of adhesion, a disrupted actin cytoskeleton, and a cytokinesis defect. How SadA mediates these phenotypes is unknown. This work addresses the mechanism of SadA function, demonstrating an important role for the C-terminal cytoplasmic tail in SadA function. We found that a SadA tailless mutant was unable to rescue the sadA adhesion deficiency, and overexpression of the SadA tail domain reduced adhesion in wild-type cells. We also show that SadA is closely associated with the actin cytoskeleton. Mutagenesis studies suggested that four serine residues in the tail, S924/S925 and S940/S941, may regulate association of SadA with the actin cytoskeleton. Glutathione S-transferase pull-down assays identified at least one likely interaction partner of the SadA tail, cortexillin I, a known actin bundling protein. Thus, our data demonstrate an important role for the carboxy-terminal cytoplasmic tail in SadA function and strongly suggest that a phosphorylation event in this tail regulates an interaction with cortexillin I. Based on our data, we propose a model for the function of SadA.
Figures

References
-
- Alibaud L., Cosson P., Benghezal M. 2003. Dictyostelium discoideum transformation by oscillating electric field electroporation. Biotechniques 35:78–80 - PubMed
-
- Appella E., Weber I. T., Blasi F. 1988. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 231:1–4 - PubMed
-
- Baldauf S. L. 2003. The deep roots of eukaryotes. Science 300:1703–1706 - PubMed
-
- Balla S., et al. 2006. Minimotif Miner: a tool for investigating protein function. Nat. Methods 3:175–177 - PubMed
-
- Blom N., Gammeltoft S., Brunak S. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351–1362 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

