Establishment of cell-cell cross talk in the epididymis: control of luminal acidification
- PMID: 21441423
- PMCID: PMC3753098
- DOI: 10.2164/jandrol.111.012971
Establishment of cell-cell cross talk in the epididymis: control of luminal acidification
Abstract
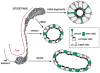
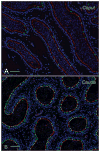
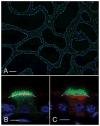
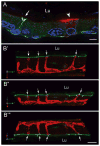
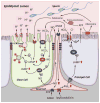
Male infertility is often caused by sperm that have low motility and interact poorly with the oocyte. Spermatozoa acquire these crucial functions in the epididymis. A low luminal bicarbonate (HCO(3)(-)) concentration and low pH keep sperm quiescent during their maturation and storage in this organ. This review describes how epididymal epithelial cells work in a concerted manner, together with spermatozoa, to establish and maintain this acidic luminal environment. Clear cells express the proton-pumping ATPase (V-ATPase) in their apical membrane and actively secrete protons. HCO(3)(-) induces V-ATPase accumulation in apical microvilli in clear cells via HCO(3)(-)-sensitive adenylyl cyclase-dependent cAMP production. HCO(3)(-) is secreted from principal cells following basolateral stimulation, to transiently "prime" spermatozoa before ejaculation. Luminal ATP and adenosine also induce V-ATPase apical accumulation in clear cells via activation of P2 and P1 receptors, respectively. ATP is released into the lumen from sperm and principal cells and is then metabolized into adenosine by local nucleotidases. In addition, the V-ATPase is regulated by luminal angiotensin II via activation of basal cells, which can extend narrow body projections that cross the tight junction barrier. Basal cells then secrete nitric oxide, which diffuses out to stimulate proton secretion in clear cells via activation of the cGMP pathway. Thus, an elaborate communication network is present between principal cells and clear cells, and between basal cells and clear cells, to control luminal acidification. Monitoring and decoding these "intercellular conversations" will help define pathophysiologic mechanisms underlying male infertility.
Figures


References
-
- Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid. II. Interaction of pH and a quiescence factor. Biol Reprod. 1984;30:926–935. - PubMed
-
- Aitken RJ. Sperm function tests and fertility. Int J Androl. 2006;29:69–75. discussion 105–108. - PubMed
-
- Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem. 2010;285:24676–24685. - PMC - PubMed
-
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J Biol Chem. 2005;280:8452–8463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
