Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect
- PMID: 21441455
- PMCID: PMC3726212
- DOI: 10.4049/jimmunol.1002932
Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect
Abstract
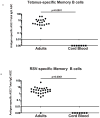
Surges of serum Abs after immunization and infection are highly specific for the offending Ag, and recent studies demonstrate that vaccines induce transient increases in circulating Ab-secreting cells (ASCs). These ASCs are highly enriched but not universally specific for the immunizing Ag, suggesting that a fraction of these ASCs could arise from polyclonal bystander stimulation of preexisting memory cells to unrelated Ags. This model is proposed to explain maintenance of long-lived serological memory in the absence of Ag exposure. To test this model, we measure the ability of respiratory syncytial virus and influenza virus infection or immunizations to influenza virus, tetanus toxoid, hepatitis B Ag, and human papillomavirus to stimulate bystander memory cells specific for other major environmental Ags that represent a large fraction of the preexisting memory B compartment. Bystander or nonspecific ASC responses to respiratory syncytial virus and tetanus could not be detected above the background levels in healthy adults, despite the presence of circulating memory B cells specific for the corresponding Ags. Nonspecific ASC responses in the healthy subjects and cord blood samples were similar. In contrast, both vaccination and infection induce massive expansion of circulating Ag-specific ASCs without significant increases in the frequencies of ASCs against unrelated Ags. Hence, nonspecific stimulation of memory B cells is unlikely to contribute to the mechanisms of long-term serological memory against major human pathogens. Additionally, high specificity of circulating ASCs after antigenic challenge highlights the diagnostic value of interrogating ASCs as an ideal single-time-point diagnostic immune surrogate for serology during acute infection.
Conflict of interest statement
Figures






References
-
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–999. - PubMed
-
- Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. - PubMed
-
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI049660/AI/NIAID NIH HHS/United States
- U01AI045969/AI/NIAID NIH HHS/United States
- U19 AI056390/AI/NIAID NIH HHS/United States
- K23 AI67501/AI/NIAID NIH HHS/United States
- U01 AI045969/AI/NIAID NIH HHS/United States
- N01 AI050029/AI/NIAID NIH HHS/United States
- K23 AI067501/AI/NIAID NIH HHS/United States
- U10AI056390/AI/NIAID NIH HHS/United States
- R01 HL069409/HL/NHLBI NIH HHS/United States
- HHSN266200500030C/AI/NIAID NIH HHS/United States
- N01-AI50029/AI/NIAID NIH HHS/United States
- AI056390-06S2/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

