Unexpected diversity of chlorite dismutases: a catalytically efficient dimeric enzyme from Nitrobacter winogradskyi
- PMID: 21441524
- PMCID: PMC3133159
- DOI: 10.1128/JB.01262-10
Unexpected diversity of chlorite dismutases: a catalytically efficient dimeric enzyme from Nitrobacter winogradskyi
Abstract
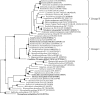
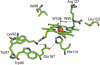
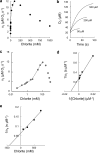
Chlorite dismutase (Cld) is a unique heme enzyme catalyzing the conversion of ClO(2)(-) to Cl(-) and O(2). Cld is usually found in perchlorate- or chlorate-reducing bacteria but was also recently identified in a nitrite-oxidizing bacterium of the genus Nitrospira. Here we characterized a novel Cld-like protein from the chemolithoautotrophic nitrite oxidizer Nitrobacter winogradskyi which is significantly smaller than all previously known chlorite dismutases. Its three-dimensional (3D) crystal structure revealed a dimer of two identical subunits, which sharply contrasts with the penta- or hexameric structures of other chlorite dismutases. Despite a truncated N-terminal domain in each subunit, this novel enzyme turned out to be a highly efficient chlorite dismutase (K(m) = 90 μM; k(cat) = 190 s(-1); k(cat)/K(m) = 2.1 × 10(6) M(-1) s(-1)), demonstrating a greater structural and phylogenetic diversity of these enzymes than was previously known. Based on comparative analyses of Cld sequences and 3D structures, signature amino acid residues that can be employed to assess whether uncharacterized Cld-like proteins may have a high chlorite-dismutating activity were identified. Interestingly, proteins that contain all these signatures and are phylogenetically closely related to the novel-type Cld of N. winogradskyi exist in a large number of other microbes, including other nitrite oxidizers.
Figures
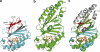
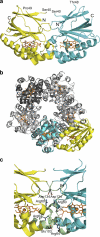
References
-
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

