External quality assessment for KRAS testing is needed: setup of a European program and report of the first joined regional quality assessment rounds
- PMID: 21441573
- PMCID: PMC3228116
- DOI: 10.1634/theoncologist.2010-0429
External quality assessment for KRAS testing is needed: setup of a European program and report of the first joined regional quality assessment rounds
Abstract
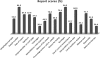
The use of epidermal growth factor receptor-targeting antibodies in metastatic colorectal cancer has been restricted to patients with wild-type KRAS tumors by the European Medicines Agency since 2008, based on data showing a lack of efficacy and potential harm in patients with mutant KRAS tumors. In an effort to ensure optimal, uniform, and reliable community-based KRAS testing throughout Europe, a KRAS external quality assessment (EQA) scheme was set up. The first large assessment round included 59 laboratories from eight different European countries. For each country, one regional scheme organizer prepared and distributed the samples for the participants of their own country. The samples included unstained sections of 10 invasive colorectal carcinomas with known KRAS mutation status. The samples were centrally validated by one of two reference laboratories. The laboratories were allowed to use their own preferred method for histological evaluation, DNA isolation, and mutation analysis. In this study, we analyze the setup of the KRAS scheme. We analyzed the advantages and disadvantages of the regional scheme organization by analyzing the outcome of genotyping results, analysis of tumor percentage, and written reports. We conclude that only 70% of laboratories correctly identified the KRAS mutational status in all samples. Both the false-positive and false-negative results observed negatively affect patient care. Reports of the KRAS test results often lacked essential information. We aim to further expand this program to more laboratories to provide a robust estimate of the quality of KRAS testing in Europe, and provide the basis for remedial measures and harmonization.
Conflict of interest statement
Section Editor
Section Editor
Section Editor
Reviewer “A” discloses no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures

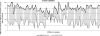
References
-
- Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. - PubMed
-
- Brink M, de Goeij AF, Weijenberg MP, et al. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003;24:703–710. - PubMed
-
- Samowitz WS, Curtin K, Schaffer D, et al. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: A population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–1197. - PubMed
-
- Barault L, Veyrie N, Jooste V, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–2259. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

