Multiple myeloma treatment strategies with novel agents in 2011: a European perspective
- PMID: 21441574
- PMCID: PMC3228121
- DOI: 10.1634/theoncologist.2010-0386
Multiple myeloma treatment strategies with novel agents in 2011: a European perspective
Abstract
The arrival of the novel agents thalidomide, bortezomib, and lenalidomide has significantly changed our approach to the management of multiple myeloma and, importantly, patient outcomes have improved. These agents have been investigated intensively in different treatment settings, providing us with data to make evidence-based decisions regarding the optimal management of patients. This review is an update to a previous summary of European treatment practices that examines new data that have been published or presented at congresses up to the end of 2010 and assesses their impact on treatment practices.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
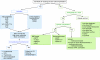
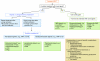
References
-
- van de Velde HJK, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–1406. - PubMed
-
- Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. - PubMed
-
- Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. - PubMed
-
- Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–2624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

