Tenascins and the importance of adhesion modulation
- PMID: 21441591
- PMCID: PMC3101840
- DOI: 10.1101/cshperspect.a004960
Tenascins and the importance of adhesion modulation
Abstract
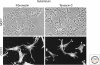
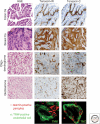
Tenascins are a family of extracellular matrix proteins that evolved in early chordates. There are four family members: tenascin-X, tenascin-R, tenascin-W, and tenascin-C. Tenascin-X associates with type I collagen, and its absence can cause Ehlers-Danlos Syndrome. In contrast, tenascin-R is concentrated in perineuronal nets. The expression of tenascin-C and tenascin-W is developmentally regulated, and both are expressed during disease (e.g., both are associated with cancer stroma and tumor blood vessels). In addition, tenascin-C is highly induced by infections and inflammation. Accordingly, the tenascin-C knockout mouse has a reduced inflammatory response. All tenascins have the potential to modify cell adhesion either directly or through interaction with fibronectin, and cell-tenascin interactions typically lead to increased cell motility. In the case of tenascin-C, there is a correlation between elevated expression and increased metastasis in several types of tumors.
Figures


References
-
- Ambort D, Brellier F, Becker-Pauly C, Stocker W, Andrejevic-Blant S, Chiquet M, Sterchi EE 2010. Specific processing of tenascin-C by the metalloprotease meprinβ neutralizes its inhibition of cell spreading. Matrix Biol 29: 31–42 - PubMed
-
- Andjus PR, Bajic A, Zhu L, Schachner M, Strata P 2005. Short-term facilitation and depression in the cerebellum: Some observations on wild-type and mutant rodents deficient in the extracellular matrix molecule tenascin C. Ann N Y Acad Sci 1048: 185–197 - PubMed
-
- Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knol C, Kirchner T, Jung A 2005. β-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene 24: 8200–8204 - PubMed
-
- Borsi L, Allemanni G, Gaggero B, Zardi L 1996. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer 66: 632–635 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
