Engineering strategies to recapitulate epithelial morphogenesis within synthetic three-dimensional extracellular matrix with tunable mechanical properties
- PMID: 21441648
- PMCID: PMC3401181
- DOI: 10.1088/1478-3975/8/2/026013
Engineering strategies to recapitulate epithelial morphogenesis within synthetic three-dimensional extracellular matrix with tunable mechanical properties
Abstract
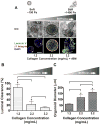
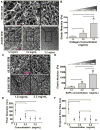
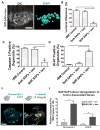
The mechanical properties (e.g. stiffness) of the extracellular matrix (ECM) influence cell fate and tissue morphogenesis and contribute to disease progression. Nevertheless, our understanding of the mechanisms by which ECM rigidity modulates cell behavior and fate remains rudimentary. To address this issue, a number of two and three-dimensional (3D) hydrogel systems have been used to explore the effects of the mechanical properties of the ECM on cell behavior. Unfortunately, many of these systems have limited application because fiber architecture, adhesiveness and/or pore size often change in parallel when gel elasticity is varied. Here we describe the use of ECM-adsorbed, synthetic, self-assembling peptide (SAP) gels that are able to recapitulate normal epithelial acini morphogenesis and gene expression in a 3D context. By exploiting the range of viscoelasticity attainable with these SAP gels, and their ability to recreate native-like ECM fibril topology with minimal variability in ligand density and pore size, we were able to reconstitute normal and tumor-like phenotypes and gene expression patterns in nonmalignant mammary epithelial cells. Accordingly, this SAP hydrogel system presents the first tunable system capable of independently assessing the interplay between ECM stiffness and multi-cellular epithelial phenotype in a 3D context.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
