The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication
- PMID: 21441896
- PMCID: PMC3098494
- DOI: 10.1038/emboj.2011.86
The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication
Abstract
Formation of aberrant protein conformers is a common pathological denominator of different neurodegenerative disorders, such as Alzheimer's disease or prion diseases. Moreover, increasing evidence indicates that soluble oligomers are associated with early pathological alterations and that oligomeric assemblies of different disease-associated proteins may share common structural features. Previous studies revealed that toxic effects of the scrapie prion protein (PrP(Sc)), a β-sheet-rich isoform of the cellular PrP (PrP(C)), are dependent on neuronal expression of PrP(C). In this study, we demonstrate that PrP(C) has a more general effect in mediating neurotoxic signalling by sensitizing cells to toxic effects of various β-sheet-rich (β) conformers of completely different origins, formed by (i) heterologous PrP, (ii) amyloid β-peptide, (iii) yeast prion proteins or (iv) designed β-peptides. Toxic signalling via PrP(C) requires the intrinsically disordered N-terminal domain (N-PrP) and the GPI anchor of PrP. We found that the N-terminal domain is important for mediating the interaction of PrP(C) with β-conformers. Interestingly, a secreted version of N-PrP associated with β-conformers and antagonized their toxic signalling via PrP(C). Moreover, PrP(C)-mediated toxic signalling could be blocked by an NMDA receptor antagonist or an oligomer-specific antibody. Our study indicates that PrP(C) can mediate toxic signalling of various β-sheet-rich conformers independent of infectious prion propagation, suggesting a pathophysiological role of the prion protein beyond of prion diseases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
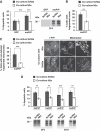
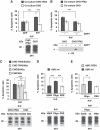

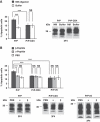
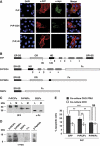


Comment in
-
Is PrP the road to ruin?EMBO J. 2011 May 18;30(10):1882-4. doi: 10.1038/emboj.2011.129. EMBO J. 2011. PMID: 21593729 Free PMC article. Review.
References
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343 - PubMed
-
- Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347 - PubMed
-
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582 - PubMed
-
- Caughey B, Baron GS (2006) Prions and their partners in crime. Nature 443: 803–810 - PubMed
-
- Chesebro B (2003) Introduction to the transmissible spongiform encephalopathies or prion diseases. Br Med Bull 66: 1–20 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

