New model systems provide insights into Myc-induced transformation
- PMID: 21441954
- PMCID: PMC3765156
- DOI: 10.1038/onc.2011.88
New model systems provide insights into Myc-induced transformation
Abstract
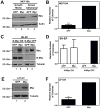
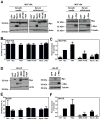
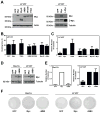
The ability of Myc to promote cellular transformation is well established; however, a better understanding of the mechanisms through which Myc mediates tumorigenesis is essential for the development of therapeutic approaches to target this potent oncoprotein. Structure-function studies in rodent fibroblast cells have provided the basis for much of our current understanding of these mechanisms. To build on these approaches, we have characterized three novel human cell line models of Myc-dependent transformation: MCF10A, SH-EP Tet21/N-Myc, and LF1/TERT/LT/ST cells. We have also evaluated Myc family proteins (c-Myc and L-Myc), a naturally occurring isoform of Myc (MycS), and a set of N-terminal domain mutants (ΔMBII, W135E, T58A) for their ability to promote anchorage-independent growth in these models. Taken together, these results provide the field with three new human cell-based models to study Myc activity, highlight the importance of cellular context, and challenge the paradigm that the ability of Myc to promote tumorigenesis is exclusively MBII-dependent.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
MYC activity is negatively regulated by a C-terminal lysine cluster.Oncogene. 2014 Feb 20;33(8):1066-72. doi: 10.1038/onc.2013.36. Epub 2013 Feb 25. Oncogene. 2014. PMID: 23435422
-
MYC phosphorylation at novel regulatory regions suppresses transforming activity.Cancer Res. 2013 Nov 1;73(21):6504-15. doi: 10.1158/0008-5472.CAN-12-4063. Epub 2013 Sep 12. Cancer Res. 2013. PMID: 24030976
-
Identification of novel targets of MYC whose transcription requires the essential MbII domain.Cell Cycle. 2006 Feb;5(3):238-41. doi: 10.4161/cc.5.3.2409. Epub 2006 Feb 1. Cell Cycle. 2006. PMID: 16434883
-
The molecular role of Myc in growth and transformation: recent discoveries lead to new insights.FASEB J. 1998 Jun;12(9):633-51. FASEB J. 1998. PMID: 9619443 Review.
-
Therapeutic aspects of c-MYC signaling in inflammatory and cancerous colonic diseases.World J Gastroenterol. 2016 Sep 21;22(35):7938-50. doi: 10.3748/wjg.v22.i35.7938. World J Gastroenterol. 2016. PMID: 27672289 Free PMC article. Review.
Cited by
-
S146L in MYC is a context-dependent activating substitution in cancer development.PLoS One. 2022 Aug 26;17(8):e0272771. doi: 10.1371/journal.pone.0272771. eCollection 2022. PLoS One. 2022. PMID: 36018850 Free PMC article.
-
Multiple direct interactions of TBP with the MYC oncoprotein.Nat Struct Mol Biol. 2019 Nov;26(11):1035-1043. doi: 10.1038/s41594-019-0321-z. Epub 2019 Nov 4. Nat Struct Mol Biol. 2019. PMID: 31686052
-
MYCN drives oncogenesis by cooperating with the histone methyltransferase G9a and the WDR5 adaptor to orchestrate global gene transcription.PLoS Biol. 2024 Mar 28;22(3):e3002240. doi: 10.1371/journal.pbio.3002240. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38547242 Free PMC article.
-
Merkel cell polyomavirus recruits MYCL to the EP400 complex to promote oncogenesis.PLoS Pathog. 2017 Oct 13;13(10):e1006668. doi: 10.1371/journal.ppat.1006668. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29028833 Free PMC article.
-
Transcriptional Control of Dendritic Cell Development.Annu Rev Immunol. 2016 May 20;34:93-119. doi: 10.1146/annurev-immunol-032713-120204. Epub 2015 Dec 23. Annu Rev Immunol. 2016. PMID: 26735697 Free PMC article. Review.
References
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–8. - PubMed
-
- Bahram F, von der Lehr N, Cetinkaya C, Larsson LG. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood. 2000;95:2104–10. - PubMed
-
- Bhatia K, Huppi K, Spangler G, Siwarski D, Iyer R, Magrath I. Point mutations in the c-Myc transactivation domain are common in Burkitt’s lymphoma and mouse plasmacytomas. Nat Genet. 1993;5:56–61. - PubMed
-
- Breit S, Schwab M. Suppression of MYC by high expression of NMYC in human neuroblastoma cells. J Neurosci Res. 1989;24:21–8. - PubMed
-
- Callus BA, PGE, JEH, AMJ, AK, JEV, et al. Cytoplasmic p53 is not required for PUMA-induced apoptosis. Cell Death and Differentiation. 2008;15:213–215. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

