Arsenic trioxide enhances the cytotoxic effect of thalidomide in a KG-1a human acute mylogenous leukemia cell line
- PMID: 21442015
- PMCID: PMC3062976
- DOI: 10.3892/ol_00000083
Arsenic trioxide enhances the cytotoxic effect of thalidomide in a KG-1a human acute mylogenous leukemia cell line
Abstract
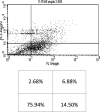
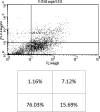
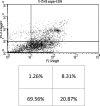
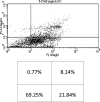
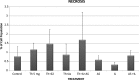
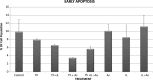
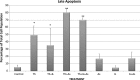
Studies have shown that thalidomide exerts modest activity as a single agent in the therapy of acute myeloid leukemia (AML). The present investigation was conducted to test the hypothesis that the cytotoxic effect of thalidomide is enhanced when properly combined with other chemotherapeutic agents. The human AML cell line KG-1a was used in this study. Cells were cultured for 48 h in the presence or absence of thalidomide, arsenic trioxide and a combination of the two substances. Results obtained indicate that thalidomide at concentrations of 1, 2 and 5 mg/l produced a dose-dependent cytotoxic effect and at 5 mg/ml resulted in late apoptosis in 49.39% of the total cell population (as compared to 5.35% in the control cells). When the cells were incubated with arsenic trioxide alone (4 µM), late apoptosis was detected in 16.97% of the total cell population. However, when cells were incubated with a combination of thalidomide (5 mg/l) and arsenic trioxide (4 µM), late apoptosis was noted to be 80.6% in the total cell population. This percentage of late apoptosis was statistically significant from that observed when cells were incubated with thalidomide alone. These findings clearly indicate that arsenic trioxide enhances the cytotoxic effects of thalidomide.
Figures
References
-
- Godwin JE, Smith SE. Acute myeloid leukemia in the older patient. Crit Rev Oncol Hematol. 2003;48:S17–S26. - PubMed
-
- Harousseuau JL. Acute myeloid leukemia in the elderly. Blood Rev. 1988;12:145–153. - PubMed
-
- Cortes JE, Kantarjian HM. Acute lymphocytic leukemia: A comprehensive review with emphasis on biology and therapy. Cancer. 1995;76:2393–2417. - PubMed
-
- Estey EH, Kantarjian H, Keating MJ. Therapy for acute myeloid leukemia. In: Hoffman R, Benz E Jr, Shattil S, Cohen H, Silberstein L, editors. Hematology: Basic Principles and Practice. 2nd edition. Churchill Livingstone; New York: 1994. pp. 1014–1028.
-
- Thomas DA, Kantarjian H, Smith TL. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
