Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid
- PMID: 21442198
- PMCID: PMC3742062
- DOI: 10.1007/s10549-011-1450-1
Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid
Abstract
Although tamoxifen can prevent primary breast cancer, few women use it as a preventive measure. A second option, raloxifene, has recently been approved. The objective of the study was to determine women's interest in tamoxifen and raloxifene after reading a decision aid (DA) describing the risks and benefits of each medication. Women with 5-year risk of breast cancer ≥ 1.66 from two large health maintenance organizations were randomized to receive a DA versus usual care. After reading an on-line DA that discussed the risks and benefits of tamoxifen and raloxifene, women completed measures of risk perception, decisional conflict, behavioral intentions, and actual behavior related to tamoxifen and raloxifene. 3 months following the intervention, 8.1% of participants had looked for additional information about breast cancer prevention drugs, and 1.8% had talked to their doctor about tamoxifen and/or raloxifene. The majority, 54.7%, had decided to not take either drug, 0.5% had started raloxifene, and none had started tamoxifen. Participants were not particularly worried about taking tamoxifen or raloxifene and did not perceive significant benefits from taking these drugs. Over 50% did not perceive a change in their risk of getting breast cancer if they took tamoxifen or raloxifene. After reading a DA about tamoxifen and raloxifene, few women were interested in taking either breast cancer prevention drug.
Figures
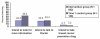


References
-
- Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Annals of Surgical Oncology. 2001;8(7):580–585. - PubMed
-
- Bober S, Hoke L, Duda R, Regan M, Tung N. Decision-making about tamoxifen in women at high risk for breast cancer: Clinical and psychological factors. Journal of Clinical Oncology. 2004;22(24):4951–4957. - PubMed
-
- Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, Nuovo J, Keyzer J, Henderson S. Preferences of women evaluating risks of tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 103(10):1996–2005. 205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

