Vestibular control of the head: possible functions of the vestibulocollic reflex
- PMID: 21442224
- PMCID: PMC4157641
- DOI: 10.1007/s00221-011-2611-5
Vestibular control of the head: possible functions of the vestibulocollic reflex
Abstract
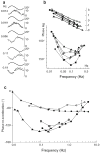
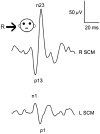
Here, we review the angular vestibulocollic reflex (VCR) focusing on its function during unexpected and voluntary head movements. Theoretically, the VCR could (1) stabilize the head in space during body movements and/or (2) dampen head oscillations that could occur as a result of the head's underdamped mechanics. The reflex appears unaffected when the simplest, trisynaptic VCR pathways are severed. The VCR's efficacy varies across species; in humans and monkeys, head stabilization is ineffective during low-frequency body movements in the yaw plan. While the appearance of head oscillations after the attenuation of semicircular canal function suggests a role in damping, this interpretation is complicated by defects in the vestibular input to other descending motor pathways such as gaze premotor circuits. Since the VCR should oppose head movements, it has been proposed that the reflex is suppressed during voluntary head motion. Consistent with this idea, vestibular-only (VO) neurons, which are possible vestibulocollic neurons, respond vigorously to passive, but not active, head rotations. Although VO neurons project to the spinal cord, their contribution to the VCR remains to be established. VCR cancelation during active head movements could be accomplished by an efference copy signal negating afferent activity related to active motion. Oscillations occurring during active motion could be eliminated by some combination of reflex actions and voluntary motor commands that take into account the head's biomechanics. A direct demonstration of the status of the VCR during active head movements is required to clarify the function of the reflex.
Figures




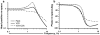



References
-
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. - PubMed
-
- Baker J, Goldberg J, Peterson B, Schor R. Oculomotor reflexes after semicircular canal plugging in cats. Brain Res. 1982;252:151–155. - PubMed
-
- Berthoz A, Anderson JH. Frequency analysis of vestibular influence on extensor motoneurons. II. Relationship between neck and forelimb extensors. Brain Res. 1971;34:376–380. - PubMed
-
- Bilotto G, Goldberg J, Peterson BW, Wilson VJ. Dynamic properties of vestibular reflexes in the decerebrate cat. Exp Brain Res. 1982;47:343–352. - PubMed
-
- Bizzi E. Eye-head coordination. In: Brooks VB, editor. Handbook of physiology the nervous system section 1. part 2. Vol. 2. American Physiological Society; Bethesda: 1981. pp. 1321–1336.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

