A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides
- PMID: 21442711
- PMCID: PMC3159489
- DOI: 10.1002/anie.201100633
A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides
Figures


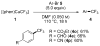
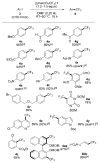


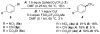

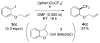
Similar articles
-
Copper-catalyzed arylation and alkenylation of polyfluoroarene C-H bonds.J Am Chem Soc. 2008 Jan 30;130(4):1128-9. doi: 10.1021/ja077862l. Epub 2008 Jan 9. J Am Chem Soc. 2008. PMID: 18181627 Free PMC article.
-
Copper-mediated trifluoromethylation using phenyl trifluoromethyl sulfoxide.Org Lett. 2015 Jan 16;17(2):298-301. doi: 10.1021/ol5034018. Epub 2014 Dec 26. Org Lett. 2015. PMID: 25541645
-
Highly efficient synthesis of phenols by copper-catalyzed hydroxylation of aryl iodides, bromides, and chlorides.Org Lett. 2011 Aug 19;13(16):4340-3. doi: 10.1021/ol2016737. Epub 2011 Jul 25. Org Lett. 2011. PMID: 21786767
-
Copper catalyzed coupling of aryl chlorides, bromides and iodides with amines and amides.Chem Commun (Camb). 2009 Apr 7;(13):1715-7. doi: 10.1039/b823407h. Epub 2009 Feb 24. Chem Commun (Camb). 2009. PMID: 19294272
-
Interaction of DNA with Simple and Mixed Ligand Copper(II) Complexes of 1,10-Phenanthrolines as Studied by DNA-Fiber EPR Spectroscopy.Int J Mol Sci. 2015 Sep 21;16(9):22754-80. doi: 10.3390/ijms160922754. Int J Mol Sci. 2015. PMID: 26402668 Free PMC article. Review.
Cited by
-
Oxidative addition of an alkyl halide to form a stable Cu(III) product.Science. 2023 Sep 8;381(6662):1072-1079. doi: 10.1126/science.adg9232. Epub 2023 Sep 7. Science. 2023. PMID: 37676952 Free PMC article.
-
Coupling of Alternating Current to Transition-Metal Catalysis: Examples of Nickel-Catalyzed Cross-Coupling.J Org Chem. 2021 Jan 1;86(1):782-793. doi: 10.1021/acs.joc.0c02350. Epub 2020 Nov 13. J Org Chem. 2021. PMID: 33186048 Free PMC article.
-
Repurposing of F-gases: challenges and opportunities in fluorine chemistry.Chem Soc Rev. 2022 Jun 20;51(12):4977-4995. doi: 10.1039/d1cs01072g. Chem Soc Rev. 2022. PMID: 35616085 Free PMC article. Review.
-
Cu-catalyzed trifluoromethylation of aryl iodides with trifluoromethylzinc reagent prepared in situ from trifluoromethyl iodide.Beilstein J Org Chem. 2013 Nov 8;9:2404-9. doi: 10.3762/bjoc.9.277. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 24367406 Free PMC article.
-
Ligand-Controlled Regioselective Copper-Catalyzed Trifluoromethylation To Generate (Trifluoromethyl)allenes.Org Lett. 2015 May 15;17(10):2506-9. doi: 10.1021/acs.orglett.5b01027. Epub 2015 Apr 24. Org Lett. 2015. PMID: 25910053 Free PMC article.
References
-
- Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320–330. - PubMed
-
-
For approaches to trifluoromethylation of aryl halides, see the following and reference [3]: Cho EJ, Senecal TD, Kinzel T, Zhang Y, Watson DA, Buchwald SL. Science. 2010;328:1679–1681.Grushin VV, Marshall WJ. J Am Chem Soc. 2006;128:12644–12645.Kitazume T, Ishikawa N. Chem Lett. 1982:137–140.Monnier F, Taillefer M. Angew Chem. 2009;121:7088–7105.Angew Chem Int Ed. 2009;48:6954–6971.Wiemers DM, Burton DJ. J Am Chem Soc. 1986;108:832–834.Cottet F, Schlosser M. Eur J Org Chem. 2002:327–330.Kütt A, Movchun V, Rodima T, Dansauer T, Rusanov EB, Leito I, Kaljurand I, Koppel J, Pihl V, Koppel I, Ovsjannikov G, Toom L, Mishima M, Medebielle M, Lork E, Roschenthaler GV, Koppel IA, Kolomeitsev AA. J Org Chem. 2008;73:2607–2620.Dubinina GG, Furutachi H, Vicic DA. J Am Chem Soc. 2008;130:8600–8601.Dubinina GG, Ogikubo J, Vicic DA. Organometallics. 2008;27:6233–6235.
-
-
- Oishi M, Kondo H, Amii H. Chem Commun. 2009:1909–1911. - PubMed
-
-
For recent developments in trifluoromethylation of arenes, see: Ball ND, Kampf JW, Sanford MS. J Am Chem Soc. 2010;132:2878–2879.Wang XS, Truesdale L, Yu JQ. J Am Chem Soc. 2010;132:3648–3649.
-
-
-
For recent developments in trifluoromethylation of aryl boronic acids, see: Senecal TD, Parsons AT, Buchwald SL. J Org Chem. 2011;76:1174–1176.Chu L, Qing FL. Org Lett. 2010;12:5060–5063.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

