Afp::mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm
- PMID: 21442721
- PMCID: PMC3081534
- DOI: 10.1002/dvg.20695
Afp::mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm
Abstract
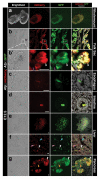
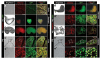
Live imaging of genetically encoded fluorescent protein reporters is increasingly being used to investigate details of the cellular behaviors that underlie the large-scale tissue rearrangements that shape the embryo. However, the majority of mouse fluorescent reporter strains are based on the green fluorescent protein (GFP). Mouse reporter strains expressing fluorescent colors other than GFP are therefore valuable for co-visualization studies with GFP, where relative positioning and relationship between two different tissues or compartments within cells are being investigated. Here, we report the generation and characterization of a transgenic Afp::mCherry mouse strain in which cis-regulatory elements from the Alpha-fetoprotein (Afp) locus were used to drive expression of the monomeric Cherry red fluorescent protein. The Afp::mCherry transgene is based on and recapitulates reporter expression of a previously described Afp::GFP strain. However, we note that perdurance of mCherry protein is not as prolonged as GFP, making the Afp::mCherry line a more faithful reporter of endogenous Afp expression. Afp::mCherry transgenic mice expressed mCherry specifically in the visceral endoderm and its derivatives, including the visceral yolk sac, gut endoderm, fetal liver, and pancreas of the embryo. The Afp::mCherry reporter was also noted to be expressed in other documented sites of Afp expression including hepatocytes as well as in pancreas, digestive tract, and brain of postnatal mice.
Copyright © 2010 Wiley-Liss, Inc.
Figures
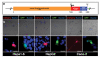
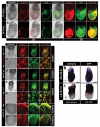
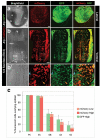
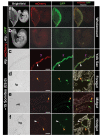
References
-
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. - PubMed
-
- Dziadek M. Modulation of alphafetoprotein synthesis in the early postimplantation mouse embryo. J Embryol Exp Morphol. 1978;46:135–146. - PubMed
-
- Dziadek M, Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J Embryol Exp Morphol. 1978;43:289–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

