A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment
- PMID: 21443232
- PMCID: PMC3520070
- DOI: 10.1021/jm101505d
A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment
Abstract
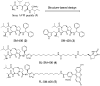
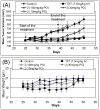
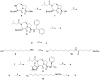
We report the discovery and characterization of SM-406 (compound 2), a potent and orally bioavailable Smac mimetic and an antagonist of the inhibitor of apoptosis proteins (IAPs). This compound binds to XIAP, cIAP1, and cIAP2 proteins with K(i) of 66.4, 1.9, and 5.1 nM, respectively. Compound 2 effectively antagonizes XIAP BIR3 protein in a cell-free functional assay, induces rapid degradation of cellular cIAP1 protein, and inhibits cancer cell growth in various human cancer cell lines. It has good oral bioavailability in mice, rats, non-human primates, and dogs, is highly effective in induction of apoptosis in xenograft tumors, and is capable of complete inhibition of tumor growth. Compound 2 is currently in phase I clinical trials for the treatment of human cancer.
Figures











References
-
- Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. - PubMed
-
- Ponder BA. Cancer genetics. Nature. 2001;411:336–341. - PubMed
-
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Salvesen GS, Duckett CS. Apoptosis: IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

