S-nitrosylation of ApoE in Alzheimer's disease
- PMID: 21443265
- PMCID: PMC3082618
- DOI: 10.1021/bi200266v
S-nitrosylation of ApoE in Alzheimer's disease
Abstract
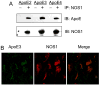
The mechanism by which apolipoprotein E (ApoE) isoforms functionally influence the risk and progression of late-onset Alzheimer's disease (LOAD) remains hitherto unknown. Herein, we present evidence that all ApoE isoforms bind to nitric oxide synthase 1 (NOS1) and that such protein-protein interaction results in S-nitrosylation of ApoE2 and ApoE3 but not ApoE4. Our structural analysis at the atomic level reveals that S-nitrosylation of ApoE2 and ApoE3 proteins may lead to conformational changes resulting in the loss of binding to low-density lipoprotein (LDL) receptors. Collectively, our data suggest that S-nitrosylation of ApoE proteins may play an important role in regulating lipid metabolism and in the pathogenesis of LOAD.
Figures


References
-
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Neurology. 1993;43:1467–1472. - PubMed
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Science. 1993;512:921–923. - PubMed
-
- Bertram L, Lill CM, Tanzi RE. Neuron. 2010;68:270–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

