Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene
- PMID: 2144364
- PMCID: PMC2935288
- DOI: 10.1126/science.2144364
Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene
Abstract
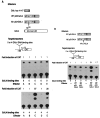
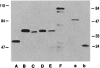
The protein encoded by the wild-type p53 proto-oncogene has been shown to suppress transformation, whereas certain mutations that alter p53 become transformation competent. Fusion proteins between p53 and the GAL4 DNA binding domain were made to anchor p53 to a DNA target sequence and to allow measurement of transcriptional activation of a reporter plasmid. The wild-type p53 stimulated transcription in this assay, but two transforming mutations in p53 were unable to act as transcriptional activators. Therefore, p53 can activate transcription, and transformation-activating mutations result in a loss of function of the p53 protein. The inability of the p53 mutant proteins to activate transcription may enable them to be transformation competent.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

