Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual
- PMID: 21444660
- PMCID: PMC3135354
- DOI: 10.1084/jem.20102608
Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual
Abstract
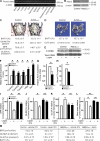
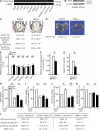
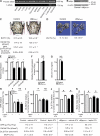
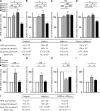
The sympathetic nervous system, whose activity is regulated by leptin signaling in the brain, is a major regulator of bone mass accrual. To determine the identity of the cell type in which the sympathetic tone signals to inhibit bone mass accrual, we performed a systematic, cell-specific analysis of the function of the β2 adrenergic receptor (Adrβ2) and various genes implicated in the pathway in the mouse. This was followed by leptin intracerebroventricular (ICV) infusion and bone histomorphometric analyses of bone parameters. We show that the sympathetic tone signals in the osteoblasts to inhibit CREB (cAMP-responsive element-binding protein) phosphorylation and thus decrease osteoblast proliferation and to promote ATF4 phosphorylation and thus increase RANKL (receptor activator of NF-κB ligand) expression, which then stimulates osteoclast differentiation. Leptin ICV infusion in various mouse models established that leptin-dependent inhibition of bone mass accrual relies on both transcriptional events taking place in osteoblasts. Thus, this study formally identifies the osteoblast as the major cell type in which the molecular events triggered by the sympathetic regulation of bone mass accrual take place. As such, it suggests that inhibiting sympathetic signaling could be beneficial in the treatment of low bone mass conditions.
Figures

References
-
- Chappard D., Palle S., Alexandre C., Vico L., Riffat G. 1987. Bone embedding in pure methyl methacrylate at low temperature preserves enzyme activities. Acta Histochem. 81:183–190 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

