Duodenal helminth infection alters barrier function of the colonic epithelium via adaptive immune activation
- PMID: 21444669
- PMCID: PMC3125859
- DOI: 10.1128/IAI.01123-10
Duodenal helminth infection alters barrier function of the colonic epithelium via adaptive immune activation
Abstract
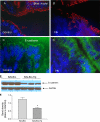
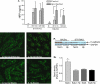
Chronic infection with intestinal helminth parasites is a major public health problem, particularly in the developing world, and can have significant effects on host physiology and the immune response to other enteric infections and antigens. The mechanisms underlying these effects are not well understood. In the current study, we investigated the impact of infection with the murine nematode parasite Heligmosomoides polygyrus, which resides in the duodenum, on epithelial barrier function in the colon. We found that H. polygyrus infection produced a significant increase in colonic epithelial permeability, as evidenced by detection of elevated serum levels of the tracer horseradish peroxidase following rectal administration. This loss of normal barrier function was associated with clear ultrastructural changes in the tight junctions of colonic epithelial cells and an alteration in the expression and distribution of the junctional protein E-cadherin. These parasite-induced abnormalities were not observed in SCID mice but did occur in SCID mice that were adoptively transferred with wild-type T cells, indicating a requirement for adaptive immunity. Furthermore, the helminth-induced increase in gut permeability was not seen in STAT6 knockout (KO) mice. Taken together, the results demonstrate that one of the mechanisms by which helminths exert their effects involves the lymphocyte- and STAT6-dependent breakdown of the intestinal epithelial barrier. This increase in epithelial permeability may facilitate the movement of lumenal contents across the mucosa, thus helping to explain how helminth infection can alter the immune response to enteric antigens.
Figures




Similar articles
-
Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis.J Immunol. 2013 Aug 15;191(4):1927-34. doi: 10.4049/jimmunol.1201457. Epub 2013 Jul 12. J Immunol. 2013. PMID: 23851695 Free PMC article.
-
Helminth-induced regulation of T-cell transfer colitis requires intact and regulated T cell Stat6 signaling in mice.Eur J Immunol. 2021 Feb;51(2):433-444. doi: 10.1002/eji.201848072. Epub 2020 Nov 18. Eur J Immunol. 2021. PMID: 33067820
-
Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice.Infect Immun. 2005 Sep;73(9):5468-81. doi: 10.1128/IAI.73.9.5468-5481.2005. Infect Immun. 2005. PMID: 16113263 Free PMC article.
-
MicroRNA-mediated regulation of immune responses to intestinal helminth infections.Parasite Immunol. 2017 Feb;39(2). doi: 10.1111/pim.12406. Parasite Immunol. 2017. PMID: 27977850 Review.
-
Immune modulation and modulators in Heligmosomoides polygyrus infection.Exp Parasitol. 2012 Sep;132(1):76-89. doi: 10.1016/j.exppara.2011.08.011. Epub 2011 Aug 22. Exp Parasitol. 2012. PMID: 21875581 Free PMC article. Review.
Cited by
-
Dietary Supplementation With Chinese Herbal Residues or Their Fermented Products Modifies the Colonic Microbiota, Bacterial Metabolites, and Expression of Genes Related to Colon Barrier Function in Weaned Piglets.Front Microbiol. 2018 Dec 21;9:3181. doi: 10.3389/fmicb.2018.03181. eCollection 2018. Front Microbiol. 2018. PMID: 30627122 Free PMC article.
-
Association of enteric parasitic infections with intestinal inflammation and permeability in asymptomatic infants of São Tomé Island.Pathog Glob Health. 2017 May;111(3):116-127. doi: 10.1080/20477724.2017.1299831. Epub 2017 Mar 10. Pathog Glob Health. 2017. PMID: 28279129 Free PMC article.
-
Relationships Between Gastrointestinal Parasite Infections and the Fecal Microbiome in Free-Ranging Western Lowland Gorillas.Front Microbiol. 2018 Jun 15;9:1202. doi: 10.3389/fmicb.2018.01202. eCollection 2018. Front Microbiol. 2018. PMID: 29963018 Free PMC article.
-
Development of fatal intestinal inflammation in MyD88 deficient mice co-infected with helminth and bacterial enteropathogens.PLoS Negl Trop Dis. 2014 Jul 10;8(7):e2987. doi: 10.1371/journal.pntd.0002987. eCollection 2014 Jul. PLoS Negl Trop Dis. 2014. PMID: 25010669 Free PMC article.
-
Moderate Hypothermia Provides Better Protection of the Intestinal Barrier than Deep Hypothermia during Circulatory Arrest in a Piglet Model: A Microdialysis Study.PLoS One. 2016 Sep 29;11(9):e0163684. doi: 10.1371/journal.pone.0163684. eCollection 2016. PLoS One. 2016. PMID: 27685257 Free PMC article.
References
-
- Berin M., et al. 1998. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J. Immunol. 161:2561–2566 - PubMed
-
- Bryant V. 1973. The life cycle of Nematospiroides dubius, Baylis 1926 (Nematoda: Heligmosomidae). J. Helminthol. 47:263–268 - PubMed
-
- Ceponis P. J. M., Botelho F., Richards C. D., McKay D. M. 2000. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 275:29132–29137 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

