Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy
- PMID: 21444780
- PMCID: PMC3076884
- DOI: 10.1073/pnas.1009932108
Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy
Abstract
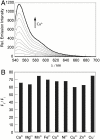
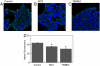
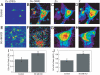
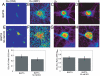
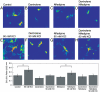
Dynamic fluxes of s-block metals like potassium, sodium, and calcium are of broad importance in cell signaling. In contrast, the concept of mobile transition metals triggered by cell activation remains insufficiently explored, in large part because metals like copper and iron are typically studied as static cellular nutrients and there are a lack of direct, selective methods for monitoring their distributions in living cells. To help meet this need, we now report Coppersensor-3 (CS3), a bright small-molecule fluorescent probe that offers the unique capability to image labile copper pools in living cells at endogenous, basal levels. We use this chemical tool in conjunction with synchotron-based microprobe X-ray fluorescence microscopy (XRFM) to discover that neuronal cells move significant pools of copper from their cell bodies to peripheral processes upon their activation. Moreover, further CS3 and XRFM imaging experiments show that these dynamic copper redistributions are dependent on calcium release, establishing a link between mobile copper and major cell signaling pathways. By providing a small-molecule fluorophore that is selective and sensitive enough to image labile copper pools in living cells under basal conditions, CS3 opens opportunities for discovering and elucidating functions of copper in living systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. - PubMed
-
- Debanne D. Information processing in the axon. Nat Rev Neurosci. 2004;5:304–316. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. - PubMed
-
- Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994.
-
- Gray HB, Stiefel EI, Valentine JS, Bertini I. Biological Inorganic Chemistry. Mill Valley, CA: University Science Books; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

