Shortening infusion time for high-dose methotrexate alters antileukemic effects: a randomized prospective clinical trial
- PMID: 21444869
- PMCID: PMC3107765
- DOI: 10.1200/JCO.2010.32.5340
Shortening infusion time for high-dose methotrexate alters antileukemic effects: a randomized prospective clinical trial
Abstract
Purpose: To determine whether shortening the infusion duration of high-dose methotrexate (HDMTX; 1 g/m(2)) affects the in vivo accumulation of active methotrexate polyglutamates (MTXPG(1-7)) in leukemia cells and whether this differs among major acute lymphoblastic leukemia (ALL) subtypes.
Methods: From June 2000 through October 2007, 356 children with ALL were randomly assigned to receive initial single-agent treatment with HDMTX (1 g/m(2)) as either a 24-hour infusion or a 4-hour infusion at two pediatric hospitals in the United States. The primary outcome measures were the accumulation of MTXPG(1-7) in leukemia cells and the antileukemic effects (eg, inhibition of de novo purine synthesis in bone marrow ALL cells, and decrease in circulating ALL cells).
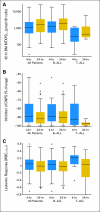
Results: The 24-hour infusion resulted in significantly higher amounts of MTXPG(1-7) in bone marrow leukemia cells (median: 1,695 v 1,150 pmol/10(9) cells, P = .0059), and better antileukemic effects. The 24-hour infusion had the greatest effect on MTXPG(1-7) accumulation in hyperdiploid ALL (median: 3,919 v 2,417 pmol/10(9) cells, P = .0038); T-cell ALL exhibited smaller differences in MTXPG(1-7) but greater antileukemic effects with the longer infusion (median decrease in leukemia cells: 88.4% v 51.8%, P = .0075). In contrast, infusion duration had no significant impact on MTXPG(1-7) accumulation or antileukemic effects in ALL with the t(12;21)/(ETV6-RUNX1) chromosomal translocation.
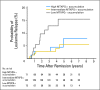
Conclusion: Shortening the infusion time of HDMTX reduces accumulation of active methotrexate in leukemia cells and decreases antileukemic effects, with differing consequences among major ALL subtypes.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Are we getting closer to using methotrexate in an optimal manner?J Clin Oncol. 2011 Sep 1;29(25):3493-4; author reply 3494-5. doi: 10.1200/JCO.2011.37.0312. Epub 2011 Aug 1. J Clin Oncol. 2011. PMID: 21810689 No abstract available.
References
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. - PubMed
-
- Conter V, Valsecchi MG, Silvestri D, et al. Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: A multicentre randomised trial. Lancet. 2007;369:123–131. - PubMed
-
- Dordelmann M, Reiter A, Zimmermann M, et al. Intermediate dose methotrexate is as effective as high dose methotrexate in preventing isolated testicular relapse in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1998;20:444–450. - PubMed
-
- Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium protocol 91-01. Blood. 2001;97:1211–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

