Retromer terminates the generation of cAMP by internalized PTH receptors
- PMID: 21445058
- PMCID: PMC3079799
- DOI: 10.1038/nchembio.545
Retromer terminates the generation of cAMP by internalized PTH receptors
Abstract
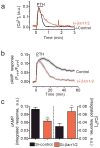
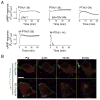
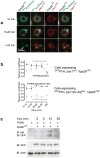
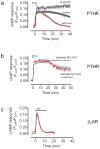
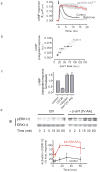
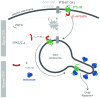
The generation of cAMP by G protein-coupled receptors (GPCRs) and its termination are currently thought to occur exclusively at the plasma membrane of cells. Under existing models of receptor regulation, this signal is primarily restricted by desensitization of the receptors through their binding to β-arrestins. However, this paradigm is not consistent with recent observations that the parathyroid hormone receptor type 1 (PTHR) continues to stimulate cAMP production even after receptor internalization, as β-arrestins are known to rapidly bind and internalize activated PTHR. Here we show that binding to β-arrestin1 prolongs rather than terminates the generation of cAMP by PTHR, and that cAMP generation correlates with the persistence of arrestin-receptor complexes on endosomes. PTHR signaling is instead turned off by the retromer complex, which regulates the movement of internalized receptor from endosomes to the Golgi apparatus. Thus, binding by the retromer complex regulates the sustained generation of cAMP triggered by an internalized GPCR.
Conflict of interest statement
The authors have no competing financial interests to disclose.
Figures
Comment in
-
Signaling: retromer arrests receptor on the run.Nat Chem Biol. 2011 May;7(5):251-2. doi: 10.1038/nchembio.564. Nat Chem Biol. 2011. PMID: 21502946 Free PMC article.
References
-
- Perry SJ, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–6. - PubMed
-
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–34. - PubMed
-
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

