Regulation of cellular senescence by the essential caveolar component PTRF/Cavin-1
- PMID: 21445100
- PMCID: PMC3193497
- DOI: 10.1038/cr.2011.56
Regulation of cellular senescence by the essential caveolar component PTRF/Cavin-1
Abstract
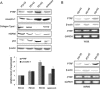
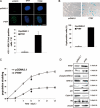
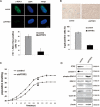
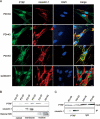
Polymerase I and transcript release factor (PTRF, also known as Cavin-1) is an essential component in the biogenesis and function of caveolae. Here, we show that PTRF expression is increased in senescent human fibroblasts. Importantly, overexpression of PTRF induced features characteristic of cellular senescence, whereas reduced PTRF expression extended the cellular replicative lifespan. Interestingly, we found that PTRF localized primarily to the nuclei of young and quiescent WI-38 human fibroblasts, but translocated to the cytosol and plasma membrane during cellular senescence. Furthermore, electron microscopic analysis demonstrated an increased number of caveolar structures in senescent and PTRF-transfected WI-38 cells. Our data suggest that the role of PTRF in cellular senescence is dependent on its targeting to caveolae and its interaction with caveolin-1, which appeared to be regulated by the phosphorylation of PTRF. Taken together, our findings identify PTRF as a novel regulator of cellular senescence that acts through the p53/p21 and caveolar pathways.
Figures



Similar articles
-
Caveolin-1 and polymerase I and transcript release factor: new players in insulin-like growth factor-I receptor signaling.J Endocrinol Invest. 2013 Mar;36(3):204-8. doi: 10.3275/8848. Epub 2013 Feb 12. J Endocrinol Invest. 2013. PMID: 23404184 Review.
-
Polymerase I and transcript release factor (PTRF)/cavin-1 is a novel regulator of stress-induced premature senescence.J Biol Chem. 2011 Aug 19;286(33):28657-28661. doi: 10.1074/jbc.C111.235119. Epub 2011 Jun 24. J Biol Chem. 2011. PMID: 21705337 Free PMC article.
-
Increased polymerase I and transcript release factor (Cavin-1) expression attenuates platelet-derived growth factor receptor signalling in senescent human fibroblasts.Clin Exp Pharmacol Physiol. 2014 Mar;41(3):169-73. doi: 10.1111/1440-1681.12202. Clin Exp Pharmacol Physiol. 2014. PMID: 24471649
-
Cavin 1 function does not follow caveolar morphology.Am J Physiol Cell Physiol. 2015 Jun 15;308(12):C1023-30. doi: 10.1152/ajpcell.00329.2014. Epub 2015 Apr 22. Am J Physiol Cell Physiol. 2015. PMID: 25904680
-
PTRF triggers a cave in.Cell. 2008 Jan 11;132(1):23-4. doi: 10.1016/j.cell.2007.12.021. Cell. 2008. PMID: 18191216 Review.
Cited by
-
Caveolin-1 and polymerase I and transcript release factor: new players in insulin-like growth factor-I receptor signaling.J Endocrinol Invest. 2013 Mar;36(3):204-8. doi: 10.3275/8848. Epub 2013 Feb 12. J Endocrinol Invest. 2013. PMID: 23404184 Review.
-
Integrated profiling identifies caveolae-associated protein 1 as a prognostic biomarker of malignancy in glioblastoma patients.CNS Neurosci Ther. 2019 Mar;25(3):343-354. doi: 10.1111/cns.13072. Epub 2018 Oct 11. CNS Neurosci Ther. 2019. PMID: 30311408 Free PMC article.
-
Caveola-forming proteins caveolin-1 and PTRF in prostate cancer.Nat Rev Urol. 2013 Sep;10(9):529-36. doi: 10.1038/nrurol.2013.168. Epub 2013 Aug 13. Nat Rev Urol. 2013. PMID: 23938946 Review.
-
Cavin-1 and Caveolin-1 are both required to support cell proliferation, migration and anchorage-independent cell growth in rhabdomyosarcoma.Lab Invest. 2015 Jun;95(6):585-602. doi: 10.1038/labinvest.2015.45. Epub 2015 Mar 30. Lab Invest. 2015. PMID: 25822667
-
Downregulation of Cavin-1 Expression via Increasing Caveolin-1 Degradation Prompts the Proliferation and Migration of Vascular Smooth Muscle Cells in Balloon Injury-Induced Neointimal Hyperplasia.J Am Heart Assoc. 2017 Jul 27;6(8):e005754. doi: 10.1161/JAHA.117.005754. J Am Heart Assoc. 2017. PMID: 28751541 Free PMC article.
References
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. - PubMed
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
-
- Hayflick L. Theories of biological aging. Exp Gerontol. 1985;20:145–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

