Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo
- PMID: 21445239
- PMCID: PMC3060173
- DOI: 10.1371/journal.ppat.1001318
Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo
Abstract
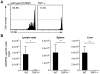
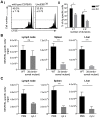
Malaria-protective CD8+ T cells specific for the circumsporozoite (CS) protein are primed by dendritic cells (DCs) after sporozoite injection by infected mosquitoes. The primed cells then eliminate parasite liver stages after recognizing the CS epitopes presented by hepatocytes. To define the in vivo processing of CS by DCs and hepatocytes, we generated parasites carrying a mutant CS protein containing the H-2K(b) epitope SIINFEKL, and evaluated the T cell response using transgenic and mutant mice. We determined that in both DCs and hepatocytes CS epitopes must reach the cytosol and use the TAP transporters to access the ER. Furthermore, we used endosomal mutant (3d) and cytochrome c treated mice to address the role of cross-presentation in the priming and effector phases of the T cell response. We determined that in DCs, CS is cross-presented via endosomes while, conversely, in hepatocytes protein must be secreted directly into the cytosol. This suggests that the main targets of protective CD8+ T cells are parasite proteins exported to the hepatocyte cytosol. Surprisingly, however, secretion of the CS protein into hepatocytes was not dependent upon parasite-export (Pexel/VTS) motifs in this protein. Together, these results indicate that the presentation of epitopes to CD8+ T cells follows distinct pathways in DCs when the immune response is induced and in hepatocytes during the effector phase.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

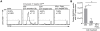
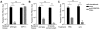
References
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. - PubMed
-
- Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. - PubMed
-
- Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, et al. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. - PubMed
-
- Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

