Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomised controlled trial
- PMID: 21445287
- PMCID: PMC3061857
- DOI: 10.1371/journal.pone.0014771
Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomised controlled trial
Abstract
Background: The uncertainty surrounding dietary requirements for selenium (Se) is partly due to limitations in biomarkers of Se status that are related to health outcomes. In this study we determined the effect of different doses and forms of Se on gene expression of selenoprotein S (SEPS1), selenoprotein W (SEPW1) and selenoprotein R (SEPR), and responses to an immune function challenge, influenza vaccine, were measured in order to identify functional markers of Se status.
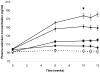
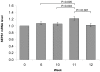
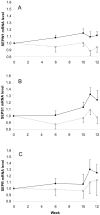
Methods and findings: A 12 week human dietary intervention study was undertaken in 119 volunteers who received placebo, 50, 100 or 200 µg/day Se-enriched yeast (Se-yeast) or meals containing unenriched or Se-enriched onions (50 µg/day). Gene expression was quantified in RNA samples extracted from human peripheral blood mononuclear cells (PBMC's) using quantitative RT-PCR. There was a significant increase in SEPW1 mRNA in the Se-enriched onion group (50 µg/day) compared with the unenriched onion group. SEPR and SEPW1 did not change significantly over the duration of the supplementation period in the control or Se-yeast groups, except at week 10 when SEPW1 mRNA levels were significantly lower in the 200 µg/day Se-yeast group compared to the placebo group. Levels of SEPS1 mRNA increased significantly 7 days after the influenza vaccine challenge, the magnitude of the increase in SEPS1 gene expression was dose-dependent, with a significantly greater response with higher Se supplementation.
Conclusions: This novel finding provides preliminary evidence for a role of SEPS1 in the immune response, and further supports the relationship between Se status and immune function.
Trial registration: ClinicalTrials.gov [NCT00279812].
Conflict of interest statement
Figures

Similar articles
-
Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial.Am J Clin Nutr. 2010 Apr;91(4):923-31. doi: 10.3945/ajcn.2009.28169. Epub 2010 Feb 24. Am J Clin Nutr. 2010. PMID: 20181815 Free PMC article. Clinical Trial.
-
The influence of selenium-enriched milk proteins and selenium yeast on plasma selenium levels and rectal selenoprotein gene expression in human subjects.Br J Nutr. 2011 Aug;106(4):572-82. doi: 10.1017/S0007114511000420. Epub 2011 Mar 30. Br J Nutr. 2011. PMID: 21450115 Clinical Trial.
-
Effect of Inorganic Dietary Selenium Supplementation on Selenoprotein and Lipid Metabolism Gene Expression Patterns in Liver and Loin Muscle of Growing Lambs.Biol Trace Elem Res. 2016 Aug;172(2):336-345. doi: 10.1007/s12011-015-0592-0. Epub 2015 Dec 23. Biol Trace Elem Res. 2016. PMID: 26701332 Free PMC article.
-
Selenium, selenoproteins and human health: a review.Public Health Nutr. 2001 Apr;4(2B):593-9. doi: 10.1079/phn2001143. Public Health Nutr. 2001. PMID: 11683552 Review.
-
Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity.J Trace Elem Med Biol. 1995 Jul;9(2):65-73. doi: 10.1016/S0946-672X(11)80013-1. J Trace Elem Med Biol. 1995. PMID: 8825978 Review.
Cited by
-
Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: Advice for healthcare professionals.Trends Food Sci Technol. 2020 Nov;105:186-199. doi: 10.1016/j.tifs.2020.09.001. Epub 2020 Sep 12. Trends Food Sci Technol. 2020. PMID: 33519086 Free PMC article. Review.
-
Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi.Sci Rep. 2011;1:72. doi: 10.1038/srep00072. Epub 2011 Aug 23. Sci Rep. 2011. PMID: 22355591 Free PMC article.
-
Association of Gpx1 fluctuation in cell cycle progression.In Vitro Cell Dev Biol Anim. 2019 Feb;55(2):94-103. doi: 10.1007/s11626-018-00314-3. Epub 2019 Jan 10. In Vitro Cell Dev Biol Anim. 2019. PMID: 30632027
-
Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults.Clin Nutr. 2017 Apr;36(2):407-415. doi: 10.1016/j.clnu.2015.12.003. Epub 2015 Dec 24. Clin Nutr. 2017. PMID: 26803169 Free PMC article. Clinical Trial.
-
Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: A review.Trends Food Sci Technol. 2021 Apr;110:66-77. doi: 10.1016/j.tifs.2021.01.069. Epub 2021 Feb 4. Trends Food Sci Technol. 2021. PMID: 33558789 Free PMC article. Review.
References
-
- Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–268. - PubMed
-
- Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. - PubMed
-
- Combs GF., Jr Impact of selenium and cancer-prevention findings on the nutrition-health paradigm. Nutr Cancer. 2001;40:6–11. - PubMed
-
- Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, et al. Research report. WCRF/AICR Expert Report. Washington: American Institute for Cancer Research; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. pp. 109–11.
-
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

