A novel role of RASSF9 in maintaining epidermal homeostasis
- PMID: 21445300
- PMCID: PMC3061870
- DOI: 10.1371/journal.pone.0017867
A novel role of RASSF9 in maintaining epidermal homeostasis
Abstract
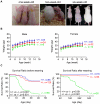
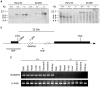
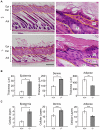
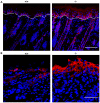
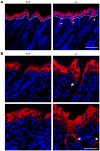
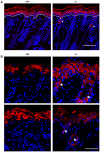
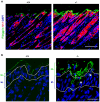
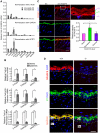
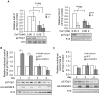
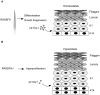
The physiological role of RASSF9, a member of the Ras-association domain family (RASSF), is currently unclear. Here, we report a mouse line in which an Epstein-Barr virus Latent Membrane Protein 1 (LMP1) transgene insertion has created a 7.2-kb chromosomal deletion, which abolished RASSF9 gene expression. The RASSF9-null mice exhibited interesting phenotypes that resembled human ageing, including growth retardation, short lifespan, less subcutaneous adipose layer and alopecia. In the wild-type mice, RASSF9 is predominantly expressed in the epidermal keratinocytes of skin, as determined by quantitative reverse-transcription PCR, immunofluorescence and in situ hybridization. In contrast, RASSF9-/- mice presented a dramatic change in epithelial organization of skin with increased proliferation and aberrant differentiation as detected by bromodeoxyuridine incorporation assays and immunofluorescence analyses. Furthermore, characteristic functions of RASSF9-/- versus wild type (WT) mouse primary keratinocytes showed significant proliferation linked to a reduction of p21Cip1 expression under growth or early differentiation conditions. Additionally, in RASSF9-/- keratinocytes there was a drastic down-modulation of terminal differentiation markers, which could be rescued by infection with a recombinant adenovirus, Adv/HA-RASSF9. Our results indicate a novel and significant role of RASSF9 in epidermal homeostasis.
Conflict of interest statement
Figures




References
-
- Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796:114–128. - PubMed
-
- Sherwood V, Recino A, Jeffries A, Ward A, Chalmers AD. The N-terminal RASSF family: a new group of Ras-association-domain-containing proteins, with emerging links to cancer formation. Biochem J. 2010;425:303–311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

