Membrane potential-dependent modulation of recurrent inhibition in rat neocortex
- PMID: 21445327
- PMCID: PMC3062529
- DOI: 10.1371/journal.pbio.1001032
Membrane potential-dependent modulation of recurrent inhibition in rat neocortex
Abstract
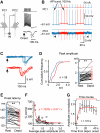
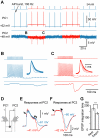
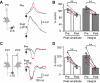
Dynamic balance of excitation and inhibition is crucial for network stability and cortical processing, but it is unclear how this balance is achieved at different membrane potentials (V(m)) of cortical neurons, as found during persistent activity or slow V(m) oscillation. Here we report that a V(m)-dependent modulation of recurrent inhibition between pyramidal cells (PCs) contributes to the excitation-inhibition balance. Whole-cell recording from paired layer-5 PCs in rat somatosensory cortical slices revealed that both the slow and the fast disynaptic IPSPs, presumably mediated by low-threshold spiking and fast spiking interneurons, respectively, were modulated by changes in presynaptic V(m). Somatic depolarization (>5 mV) of the presynaptic PC substantially increased the amplitude and shortened the onset latency of the slow disynaptic IPSPs in neighboring PCs, leading to a narrowed time window for EPSP integration. A similar increase in the amplitude of the fast disynaptic IPSPs in response to presynaptic depolarization was also observed. Further paired recording from PCs and interneurons revealed that PC depolarization increases EPSP amplitude and thus elevates interneuronal firing and inhibition of neighboring PCs, a reflection of the analog mode of excitatory synaptic transmission between PCs and interneurons. Together, these results revealed an immediate V(m)-dependent modulation of cortical inhibition, a key strategy through which the cortex dynamically maintains the balance of excitation and inhibition at different states of cortical activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Comment in
-
Finding balance in cortical networks.PLoS Biol. 2011 Mar;9(3):e1001035. doi: 10.1371/journal.pbio.1001035. Epub 2011 Mar 22. PLoS Biol. 2011. PMID: 21445331 Free PMC article. No abstract available.
Similar articles
-
Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex.J Physiol. 1996 Oct 1;496 ( Pt 1)(Pt 1):81-102. doi: 10.1113/jphysiol.1996.sp021667. J Physiol. 1996. PMID: 8910198 Free PMC article.
-
Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells.Neuron. 2007 Mar 1;53(5):735-46. doi: 10.1016/j.neuron.2007.02.012. Neuron. 2007. PMID: 17329212
-
Two distinct types of inhibition mediated by cartwheel cells in the dorsal cochlear nucleus.J Neurophysiol. 2009 Aug;102(2):1287-95. doi: 10.1152/jn.91272.2008. Epub 2009 May 27. J Neurophysiol. 2009. PMID: 19474167 Free PMC article.
-
Synaptic inhibition in the neocortex: Orchestration and computation through canonical circuits and variations on the theme.Cortex. 2020 Nov;132:258-280. doi: 10.1016/j.cortex.2020.08.015. Epub 2020 Sep 9. Cortex. 2020. PMID: 33007640 Review.
-
Layer 1 neocortex: Gating and integrating multidimensional signals.Neuron. 2024 Jan 17;112(2):184-200. doi: 10.1016/j.neuron.2023.09.041. Epub 2023 Oct 31. Neuron. 2024. PMID: 37913772 Free PMC article. Review.
Cited by
-
Dopaminergic modulation of axonal potassium channels and action potential waveform in pyramidal neurons of prefrontal cortex.J Physiol. 2013 Jul 1;591(13):3233-51. doi: 10.1113/jphysiol.2013.251058. Epub 2013 Apr 8. J Physiol. 2013. PMID: 23568892 Free PMC article.
-
Anatomy and physiology of the thick-tufted layer 5 pyramidal neuron.Front Cell Neurosci. 2015 Jun 26;9:233. doi: 10.3389/fncel.2015.00233. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26167146 Free PMC article. Review.
-
Lateral inhibition by Martinotti interneurons is facilitated by cholinergic inputs in human and mouse neocortex.Nat Commun. 2018 Oct 5;9(1):4101. doi: 10.1038/s41467-018-06628-w. Nat Commun. 2018. PMID: 30291244 Free PMC article.
-
What are the mechanisms for analogue and digital signalling in the brain?Nat Rev Neurosci. 2013 Jan;14(1):63-9. doi: 10.1038/nrn3361. Epub 2012 Nov 28. Nat Rev Neurosci. 2013. PMID: 23187813 Review.
-
Enhancement of asynchronous release from fast-spiking interneuron in human and rat epileptic neocortex.PLoS Biol. 2012;10(5):e1001324. doi: 10.1371/journal.pbio.1001324. Epub 2012 May 8. PLoS Biol. 2012. PMID: 22589699 Free PMC article.
References
-
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11:535–537. - PubMed
-
- Shu Y, Hasenstaub A, McCormick D. A. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. - PubMed
-
- Monier C, Chavane F, Baudot P, Graham L. J, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. - PubMed
-
- Wehr M, Zador A. M. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

