The intracellular threonine of amyloid precursor protein that is essential for docking of Pin1 is dispensable for developmental function
- PMID: 21445342
- PMCID: PMC3062548
- DOI: 10.1371/journal.pone.0018006
The intracellular threonine of amyloid precursor protein that is essential for docking of Pin1 is dispensable for developmental function
Abstract
Background: Processing of Aβ-precursor protein (APP) plays an important role in Alzheimer's Disease (AD) pathogenesis. Thr residue at amino acid 668 of the APP intracellular domain (AID) is highly conserved. When phosphorylated, this residue generates a binding site for Pin1. The interaction of APP with Pin1 has been involved in AD pathogenesis.
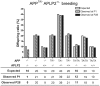
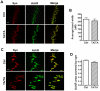
Methodology/principal findings: To dissect the functions of this sequence in vivo, we created an APP knock-in allele, in which Thr(668) is replaced by an Ala (T(668)A). Doubly deficient APP/APP-like protein 2 (APLP2) mice present postnatal lethality and neuromuscular synapse defects. Previous work has shown that the APP intracellular domain is necessary for preventing early lethality and neuromuscular junctions (NMJ) defects. Crossing the T(668)A allele into the APLP2 knockout background showed that mutation of Thr(668) does not cause a defective phenotype. Notably, the T(668)A mutant APP is able to bind Mint1.
Conclusions/significance: Our results argue against an important role of the Thr(668) residue in the essential function of APP in developmental regulation. Furthermore, they indicate that phosphorylation at this residue is not functionally involved in those APP-mediated functions that prevent (NMJ) defects and early lethality in APLP2 null mice.
Conflict of interest statement
Figures

References
-
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. - PubMed
-
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases–new features and familiar faces. Science. 2001;293:1449–1454. - PubMed
-
- Haass C, De Strooper B. The presenilins in Alzheimer's disease–proteolysis holds the key. Science. 1999;286:916–919. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Hardy J. Has the amyloid cascade hypothesis for Alzheimer's disease been proved? Curr Alzheimer Res. 2006;3:71–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

