A membrane-based, high-efficiency, microfluidic debubbler
- PMID: 21445396
- PMCID: PMC12107612
- DOI: 10.1039/c1lc20089e
A membrane-based, high-efficiency, microfluidic debubbler
Abstract
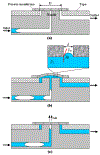
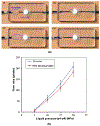
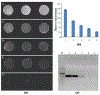
In many lab-on-chip applications, it is necessary to remove bubbles from the flow stream. Existing bubble removal strategies have various drawbacks such as low degassing efficiency, long degassing time, large dead volumes, sensitivity to surfactants, and the need for an external vacuum or pressure source. We report on a novel, simple, robust, passive, nozzle-type, membrane-based debubbler that can be readily incorporated into microfluidic devices for rapid degassing. The debubbler is particularly suitable to operate with microfluidic systems made with plastic. The debubbler consists of a hydrophobic, porous membrane that resembles a normally closed valve, which is forced open by the working fluid's pressure. To illustrate the operation of the debubbler, we describe its use in the context of a chip containing a bead array for immunoassays. Our debubbler was able to completely filter gas bubbles out of a segmented flow at rates up to 60 µl s(-1) mm(-2) of membrane area.
© The Royal Society of Chemistry 2011
Figures

References
-
- Meng DD, Cubaud T, Ho C-M and Kim C-J, J. Microelectromech. Syst, 2007, 16, 1403–1410.
-
- Leclerc E, Sakai Y and Fujii T, Biomed. Microdevices, 2003, 5, 109–114.
-
- Kim L, Vahey MD, Lee H-Y and Voldman J, Lab Chip, 2006, 6, 394–406. - PubMed
-
- Nakayama T, Hiep HM, Furui S, Yonezawa Y, Saito M, Takamura Y, Tamiya E, Anal. Bioanal. Chem, 2010, 396, 457–464. - PubMed
-
- Sohn YS, Goodey A, Anslyn EV, McDevitt JT, Shear JB, Neikirk DP, Biosens Bioelectron., 2005, 21, 303–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

