Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection
- PMID: 21445990
- PMCID: PMC3132302
- DOI: 10.1002/jor.21414
Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection
Abstract
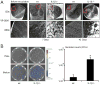
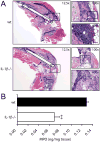
MyD88 is an adapter molecule that is used by both IL-1R and TLR family members to initiate downstream signaling and promote immune responses. Given that IL-1β is induced after Staphylococcus aureus infections and TLR2 is activated by S. aureus lipopeptides, we hypothesized that IL-1β and TLR2 contribute to MyD88-dependent protective immune responses against post-arthroplasty S. aureus infections. To test this hypothesis, we used a mouse model of a post-arthroplasty S. aureus infection to compare the bacterial burden, biofilm formation and neutrophil recruitment in IL-1β-deficient, TLR2-deficient and wild-type (wt) mice. By using in vivo bioluminescence imaging, we found that the bacterial burden in IL-1β-deficient mice was 26-fold higher at 1 day after infection and remained 3- to 10-fold greater than wt mice through day 42. In contrast, the bacterial burden in TLR2-deficient mice did not differ from wt mice. In addition, implants harvested from IL-1β-deficient mice had more biofilm formation and 14-fold higher adherent bacteria compared with those from wt mice. Finally, IL-1β-deficient mice had ∼50% decreased neutrophil recruitment to the infected postoperative joints than wt mice. Taken together, these findings suggest a mechanism by which IL-1β induces neutrophil recruitment to help control the bacterial burden and the ensuing biofilm formation in a post-surgical joint.
Copyright © 2011 Orthopaedic Research Society.
Figures

References
-
- Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. - PubMed
-
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. - PubMed
-
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. - PubMed
-
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

