FGFR1 abrogates inhibitory effect of androgen receptor concurrent with induction of androgen-receptor variants in androgen receptor-negative prostate tumor epithelial cells
- PMID: 21446013
- PMCID: PMC3513346
- DOI: 10.1002/pros.21386
FGFR1 abrogates inhibitory effect of androgen receptor concurrent with induction of androgen-receptor variants in androgen receptor-negative prostate tumor epithelial cells
Abstract
Background: Despite dramatic positive effects, there is evidence that the androgen receptor (AR) may negatively influence prostate tumor progression. Understanding the AR repressor function and how it is subverted is of particular importance in anti-androgen and AR intervention strategies.
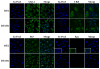
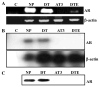
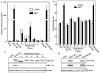
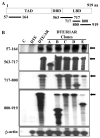
Methods: AR, resident FGFR2IIIb, and ectopic FGFR1 were expressed by transfection in the AR-negative epithelial cell line DTE that predominates in cell culture of AR-positive androgen-responsive model Dunning R3327 rat prostate tumors. Androgen-responsiveness at transcription was measured by a luciferase reporter. Cell population growth rates were assessed by cell counts, DNA synthesis, and expression of cell cycle genes. AR variants (ARVs) were assessed by immunochemistry and nuclease protection of mRNA.
Results: Expression of AR inhibited cell population growth of AR-negative DTE cells at the G1-S phase of the cell cycle. Ectopic FGFR1, but not resident FGFR2IIIb abrogated the growth inhibitory effects of AR. Appearance of ARVs was coincident with co-expression of FGFR1 and AR and abrogation of the AR-dependent inhibition of cell growth.
Conclusions: DTE cells may represent non-malignant AR-negative progenitors whose population is restricted by activation of AR in vivo. Ectopic expression of epithelial FGFR1, a common observation in tumors, overrides the inhibition of AR and thus may contribute to evolution of androgen and AR independent tumors. These results are consistent with the notion that some tumor cells are negatively restricted by AR and are unleased by androgen-deprivation or ectopic expression of FGFR1. ARV's may play a role in the bypass of the negative restrictions of AR.
Copyright © 2011 Wiley-Liss, Inc.
Figures

Similar articles
-
Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer.Endocr Relat Cancer. 2014 May 8;21(3):473-86. doi: 10.1530/ERC-13-0514. Print 2014 Jun. Endocr Relat Cancer. 2014. PMID: 24812058
-
Dominant-negative androgen receptor inhibition of intracrine androgen-dependent growth of castration-recurrent prostate cancer.PLoS One. 2012;7(1):e30192. doi: 10.1371/journal.pone.0030192. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272301 Free PMC article.
-
Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity.Prostate. 2005 Dec 1;65(4):287-98. doi: 10.1002/pros.20285. Prostate. 2005. PMID: 16015608
-
Androgen receptor action in hormone-dependent and recurrent prostate cancer.J Cell Biochem. 2006 Oct 1;99(2):362-72. doi: 10.1002/jcb.20811. J Cell Biochem. 2006. PMID: 16619264 Review.
-
AR, the cell cycle, and prostate cancer.Nucl Recept Signal. 2008 Feb 1;6:e001. doi: 10.1621/nrs.06001. Nucl Recept Signal. 2008. PMID: 18301781 Free PMC article. Review.
Cited by
-
Fibroblast Growth Factor Receptor 1 Drives the Metastatic Progression of Prostate Cancer.Eur Urol Oncol. 2022 Apr;5(2):164-175. doi: 10.1016/j.euo.2021.10.001. Epub 2021 Nov 11. Eur Urol Oncol. 2022. PMID: 34774481 Free PMC article.
-
Fibroblast Growth Factor Family in the Progression of Prostate Cancer.J Clin Med. 2019 Feb 4;8(2):183. doi: 10.3390/jcm8020183. J Clin Med. 2019. PMID: 30720727 Free PMC article. Review.
References
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. - PubMed
-
- Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29(25):3593–3604. - PubMed
-
- Mathew P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: the case for a prospective trial. Ann Oncol. 2008;19(2):395–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

