Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients
- PMID: 21446020
- PMCID: PMC3073544
- DOI: 10.1002/ana.22337
Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients
Abstract
Objective: Multiple Sclerosis (MS) is an inflammatory demyelinating disease of the human central nervous system. Although the clinical impact of gray matter pathology in MS brains is unknown, 30 to 40% of MS patients demonstrate memory impairment. The molecular basis of this memory dysfunction has not yet been investigated in MS patients.
Methods: To investigate possible mechanisms of memory impairment in MS patients, we compared morphological and molecular changes in myelinated and demyelinated hippocampi from postmortem MS brains.
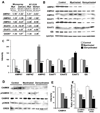
Results: Demyelinated hippocampi had minimal neuronal loss but significant decreases in synaptic density. Neuronal proteins essential for axonal transport, synaptic plasticity, glutamate neurotransmission, glutamate homeostasis, and memory/learning were significantly decreased in demyelinated hippocampi, but not in demyelinated motor cortices from MS brains.
Interpretation: Collectively, these data support hippocampal demyelination as a cause of synaptic alterations in MS patients and establish that the neuronal genes regulated by myelination reflect specific functions of neuronal subpopulations.
Copyright © 2010 American Neurological Association.
Figures



Comment in
-
Beyond axonal transection: hippocampal damage in multiple sclerosis.Ann Neurol. 2011 Mar;69(3):433-6. doi: 10.1002/ana.22409. Ann Neurol. 2011. PMID: 21446019 No abstract available.
References
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. - PubMed
-
- Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. - PubMed
-
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. - PubMed
-
- Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16:283–288. - PubMed
-
- Rao SM, Leo GJ, Aubin-Faubert P. On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol. 1989;11:699–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

