Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis
- PMID: 21446022
- PMCID: PMC3580047
- DOI: 10.1002/ana.22109
Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis
Abstract
Objective: Cerebral atrophy is a correlate of clinical progression in multiple sclerosis (MS). Mitochondria are now established to play a part in the pathogenesis of MS. Uniquely, mitochondria harbor their own mitochondrial DNA (mtDNA), essential for maintaining a healthy central nervous system. We explored mitochondrial respiratory chain activity and mtDNA deletions in single neurons from secondary progressive MS (SPMS) cases.
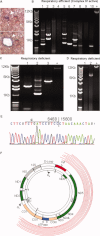
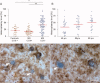
Methods: Ninety-eight snap-frozen brain blocks from 13 SPMS cases together with complex IV/complex II histochemistry, immunohistochemistry, laser dissection microscopy, long-range and real-time PCR and sequencing were used to identify and analyze respiratory-deficient neurons devoid of complex IV and with complex II activity.
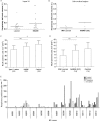
Results: The density of respiratory-deficient neurons in SPMS was strikingly in excess of aged controls. The majority of respiratory-deficient neurons were located in layer VI and immediate subcortical white matter (WM) irrespective of lesions. Multiple deletions of mtDNA were apparent throughout the gray matter (GM) in MS. The respiratory-deficient neurons harbored high levels of clonally expanded mtDNA deletions at a single-cell level. Furthermore, there were neurons lacking mtDNA-encoded catalytic subunits of complex IV. mtDNA deletions sufficiently explained the biochemical defect in the majority of respiratory-deficient neurons.
Interpretation: These findings provide evidence that neurons in MS are respiratory-deficient due to mtDNA deletions, which are extensive in GM and may be induced by inflammation. We propose induced multiple deletions of mtDNA as an important contributor to neurodegeneration in MS.
Copyright © 2010 American Neurological Association.
Figures


References
-
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989;112((pt 6)):1419–1428. - PubMed
-
- Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. - PubMed
-
- Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–254. - PubMed
-
- Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31. discussion S43–S54. - PubMed
-
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

