A galectin-3 sequence polymorphism confers TRAIL sensitivity to human breast cancer cells
- PMID: 21446041
- PMCID: PMC3164935
- DOI: 10.1002/cncr.26078
A galectin-3 sequence polymorphism confers TRAIL sensitivity to human breast cancer cells
Abstract
Background: A common polymorphism, rs4644, coding for Pro64 or His64 of the carbohydrate-binding protein galectin-3, influences the susceptibility of galectin-3 to cleavage by matrix metalloproteinases and is associated with breast cancer incidence. Because forced expression of galectin-3 in a galectin-3 null breast cancer cell line confers sensitivity to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), the authors sought to determine whether the His64/Pro64 polymorphism of galectin-3 affects the sensitivity to TRAIL.
Methods: Genomic DNA of breast cell lines was analyzed for the single nucleotide polymorphism rs4644, and cytotoxicity was determined with the MTT assay.
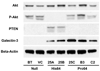
Results: When a collection of 9 breast cancer cell lines that express galectin-3 was examined for lectin, galactoside-binding, soluble, 3 (LGALS3) genotype and sensitivity to doxorubicin and TRAIL, doxorubicin sensitivity was not found to be related to LGALS3 genotype. In contrast, none of the 5 cell lines that were homozygous for Pro64 galectin-3 were found to be sensitive to TRAIL, but 2 of 2 homozygous His64 cell lines and 1 of 2 heterozygous His64 cell lines were sensitive to TRAIL. Forced expression of galectin-3 of defined genotype in galectin-3 null cells was used to more directly test the effect of the Pro64His mutation on TRAIL sensitivity. High levels of expression of His64 galectin-3 rendered BT549 cells sensitive to TRAIL and resistant to doxorubicin, but cells expressing Pro64 galectin-3 remained resistant to TRAIL and sensitive to doxorubicin.
Conclusions: The results of the current study indicate that the naturally occurring Pro64His mutation in galectin-3 increases sensitivity to death receptor-mediated apoptosis. This finding could be relevant to disparities in breast cancer outcomes across population groups, and could guide the design of future clinical trials of TRAIL-based therapies.
Copyright © 2011 American Cancer Society.
Conflict of interest statement
There are no conflicts of interest or financial disclosures for any authors.
Figures



Comment in
-
Relationship between galectin-3 expression and TRAIL sensitivity in breast cancer.Expert Rev Anticancer Ther. 2011 Aug;11(8):1193-6. doi: 10.1586/era.11.108. Expert Rev Anticancer Ther. 2011. PMID: 21916572
References
-
- Hsu DK, Liu FT. Regulation of cellular homeostasis by galectins. Glycoconj J. 2004;19:507–515. - PubMed
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F-T, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Choi JH, Chun KH, Raz A, Lotan R. Inhibition of N-(4-hydroxyphenyl)retinamide-induced apoptosis in breast cancer cells by galectin-3. Cancer Biol Ther. 2004;3:447–452. - PubMed
-
- Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, Raz A, Bresalier RS. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem. 2007;282:21337–21348. - PubMed
-
- Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its antiapoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852–6857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

