Catalyst-controlled Wacker-type oxidation of homoallylic alcohols in the absence of protecting groups
- PMID: 21446720
- PMCID: PMC3092369
- DOI: 10.1021/jo200462a
Catalyst-controlled Wacker-type oxidation of homoallylic alcohols in the absence of protecting groups
Abstract
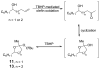
Homoallylic alcohols are oxidized to β-hydroxy ketones using a TBHP-mediated Pd-catalyzed Wacker-type oxidation. The use of a bidentate ligand, quinoline-2-oxazoline (Quinox), and TBHP((aq)) as the terminal oxidant provides good yields of the desired products with reaction times significantly reduced as compared to the Tsuji-Wacker oxidation. Additionally, bis- and tris-homoallylic alcohols are oxidized to provide cyclic peroxyketals, presumably via nucleophilic attack of the methyl ketone product.
© 2011 American Chemical Society
Figures
Similar articles
-
Wacker-type oxidation of internal alkenes using Pd(Quinox) and TBHP.J Org Chem. 2013 Feb 15;78(4):1682-6. doi: 10.1021/jo302638v. Epub 2013 Feb 6. J Org Chem. 2013. PMID: 23363387 Free PMC article.
-
A general and efficient catalyst system for a Wacker-type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates.J Am Chem Soc. 2009 May 6;131(17):6076-7. doi: 10.1021/ja901212h. J Am Chem Soc. 2009. PMID: 19364100 Free PMC article.
-
On the mechanism of the palladium-catalyzed tert-butylhydroperoxide-mediated Wacker-type oxidation of alkenes using quinoline-2-oxazoline ligands.J Am Chem Soc. 2011 Jun 1;133(21):8317-25. doi: 10.1021/ja2017043. Epub 2011 May 9. J Am Chem Soc. 2011. PMID: 21553838 Free PMC article.
-
Polyketide building blocks via diastereoselective nitrile oxide cycloadditions with homoallylic alcohols and monoprotected homoallylic diols.Chemistry. 2009 Nov 9;15(44):12065-81. doi: 10.1002/chem.200900529. Chemistry. 2009. PMID: 19780105
-
Realization of Anti-Markovnikov Selectivity in Pd-Catalyzed Oxidative Acetalization and Wacker-Type Oxidation of Terminal Alkenes.Chem Rec. 2021 Dec;21(12):3458-3469. doi: 10.1002/tcr.202100090. Epub 2021 May 21. Chem Rec. 2021. PMID: 34021681 Review.
Cited by
-
Peroxide-Mediated Wacker Oxidations for Organic Synthesis.Aldrichimica Acta. 2011;44(3):55-62. Aldrichimica Acta. 2011. PMID: 27746479 Free PMC article. No abstract available.
-
Imparting catalyst control upon classical palladium-catalyzed alkenyl C-H bond functionalization reactions.Acc Chem Res. 2012 Jun 19;45(6):874-84. doi: 10.1021/ar200236v. Epub 2011 Nov 23. Acc Chem Res. 2012. PMID: 22111756 Free PMC article.
-
Enantio- and Regioselective Palladium(II)-Catalyzed Dioxygenation of (Aza-)Alkenols.Angew Chem Int Ed Engl. 2021 Sep 27;60(40):21723-21727. doi: 10.1002/anie.202109312. Epub 2021 Sep 6. Angew Chem Int Ed Engl. 2021. PMID: 34387928 Free PMC article.
-
Insights into the Palladium(II)-Catalyzed Wacker-Type Oxidation of Styrene with Hydrogen Peroxide and tert-Butyl Hydroperoxide.ACS Catal. 2024 Jan 16;14(3):1567-1574. doi: 10.1021/acscatal.3c05630. eCollection 2024 Feb 2. ACS Catal. 2024. PMID: 38327641 Free PMC article.
-
Wacker-type oxidation of internal alkenes using Pd(Quinox) and TBHP.J Org Chem. 2013 Feb 15;78(4):1682-6. doi: 10.1021/jo302638v. Epub 2013 Feb 6. J Org Chem. 2013. PMID: 23363387 Free PMC article.
References
-
- Smidt J, Hafner W, Jira R, Sedlmeier J, Sieber R, Ruttinger R, Kojer H. Angew Chem. 1959;71:176–182.
-
- Smidt J, Hafner W, Jira R, Sieber R, Sedlmeier J, Sabel A. Angew Chem. 1962;74:93–102.
-
- Tsuji J. Synthesis. 1984:369–384.
-
- Clement WH, Selwitz CM. J Org Chem. 1964;29:241–243.
-
- Muzart J. Tetrahedron. 2007;63:7505–7521.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous