The Leukocyte Antibody Prevalence Study-II (LAPS-II): a retrospective cohort study of transfusion-related acute lung injury in recipients of high-plasma-volume human leukocyte antigen antibody-positive or -negative components
- PMID: 21446938
- PMCID: PMC3606005
- DOI: 10.1111/j.1537-2995.2011.03120.x
The Leukocyte Antibody Prevalence Study-II (LAPS-II): a retrospective cohort study of transfusion-related acute lung injury in recipients of high-plasma-volume human leukocyte antigen antibody-positive or -negative components
Abstract
Background: We used a multicenter retrospective cohort study design to evaluate whether human leukocyte antigen (HLA) antibody donor screening would reduce the risk of transfusion-related acute lung injury (TRALI) or possible TRALI.
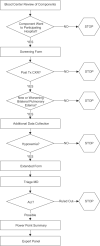
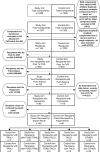
Study design and methods: In the Leukocyte Antibody Prevalence Study-II (LAPS-II), we evaluated pulmonary outcomes in recipients of 2596 plasma-rich blood components (transfusable plasma and plateletpheresis) sent to participating hospitals; half of the components were collected from anti-HLA-positive donors (study arm) and half from anti-HLA-negative donors (control arm) matched by sex, parity, and blood center. A staged medical record review process was used. Final recipient diagnosis was based on case review by a blinded expert panel of pulmonary or critical care physicians.
Results: TRALI incidence was 0.59% (seven cases) in study arm recipients versus 0.16% (two cases) in control arm recipients for an odds ratio (OR) of 3.6 (95% confidence interval [CI], 0.7-17.4; p = 0.10). For possible TRALI cases (nine study arm, eight control arm), the OR was 1.2 (95% CI, 0.4-3.0; p = 0.81), and for TRALI and possible TRALI aggregated together, it was 1.7 (95% CI, 0.7-3.7; p = 0.24). Transfusion-associated circulatory overload incidence was identical in the two arms (1.17 and 1.22%, respectively; OR, 1.0; p = 1.0).
Conclusions: TRALI incidence in recipients of anti-HLA-positive components was relatively low for a lookback study (1 in 170) and was higher than in the control arm, but did not reach significance. Based on this trend, the data are consistent with the likelihood that TRALI risk is decreased by selecting high-volume plasma components for transfusion from donors at low risk of having HLA antibodies.
© 2011 American Association of Blood Banks.
Figures


Comment in
-
The mystery of transfusion-related acute lung injury.Transfusion. 2011 Oct;51(10):2054-7. doi: 10.1111/j.1537-2995.2011.03275.x. Transfusion. 2011. PMID: 21985040 Free PMC article. No abstract available.
References
-
- Kleinman S, Grossman B, Kopko P. A national survey of transfusion-related acute lung injury risk reduction policies for platelets and plasma in the United States. Transfusion. 2010;50:1312–21. - PubMed
-
- Chapman C, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson L. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. - PubMed
-
- Engelfriet CP, Reesnik HW. International forum: measures to prevent TRALI. Vox Sang. 2007;92:258–77. - PubMed
-
- Reil A, Keller-Stanislawski B, Günay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–7. - PubMed
-
- Sachs U, Link E, Hofmann C, Wasel W, Bein G. Screening of multiparous women to avoid transfusion-related acute lung injury: a single centre experience. Transfus Med. 2008;18:348–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

