Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates
- PMID: 21448240
- PMCID: PMC3056723
- DOI: 10.1371/journal.pone.0017755
Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates
Abstract
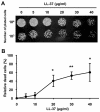
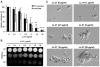
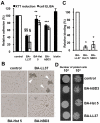
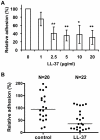
Candida albicans is the major fungal pathogen of humans. Fungal adhesion to host cells is the first step of mucosal infiltration. Antimicrobial peptides play important roles in the initial mucosal defense against C. albicans infection. LL-37 is the only member of the human cathelicidin family of antimicrobial peptides and is commonly expressed in various tissues and cells, including epithelial cells of both the oral cavity and urogenital tract. We found that, at sufficiently low concentrations that do not kill the fungus, LL-37 was still able to reduce C. albicans infectivity by inhibiting C. albicans adhesion to plastic surfaces, oral epidermoid OECM-1 cells, and urinary bladders of female BALB/c mice. Moreover, LL-37-treated C. albicans floating cells that did not adhere to the underlying substratum aggregated as a consequence of LL-37 bound to the cell surfaces. According to the results of a competition assay, the inhibitory effects of LL-37 on cell adhesion and aggregation were mediated by its preferential binding to mannan, the main component of the C. albicans cell wall, and partially by its ability to bind chitin or glucan, which underlie the mannan layer. Therefore, targeting of cell-wall carbohydrates by LL-37 provides a new strategy to prevent C. albicans infection, and LL-37 is a useful, new tool to screen for other C. albicans components involved in adhesion.
Conflict of interest statement
Figures


Similar articles
-
Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37.Int J Mol Sci. 2021 Sep 30;22(19):10633. doi: 10.3390/ijms221910633. Int J Mol Sci. 2021. PMID: 34638975 Free PMC article.
-
Characterizing the role of cell-wall β-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37.PLoS One. 2011;6(6):e21394. doi: 10.1371/journal.pone.0021394. Epub 2011 Jun 21. PLoS One. 2011. PMID: 21713010 Free PMC article.
-
Responses of Candida albicans to the human antimicrobial peptide LL-37.J Microbiol. 2014 Jul;52(7):581-9. doi: 10.1007/s12275-014-3630-2. Epub 2014 May 30. J Microbiol. 2014. PMID: 24879350
-
Face/Off: The Interchangeable Side of Candida Albicans.Front Cell Infect Microbiol. 2020 Jan 28;9:471. doi: 10.3389/fcimb.2019.00471. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32047726 Free PMC article. Review.
-
Targeting adhesion in fungal pathogen Candida albicans.Future Med Chem. 2021 Feb;13(3):313-334. doi: 10.4155/fmc-2020-0052. Epub 2020 Jun 22. Future Med Chem. 2021. PMID: 32564615 Review.
Cited by
-
Proteinous Components of Neutrophil Extracellular Traps Are Arrested by the Cell Wall Proteins of Candida albicans during Fungal Infection, and Can Be Used in the Host Invasion.Cells. 2021 Oct 13;10(10):2736. doi: 10.3390/cells10102736. Cells. 2021. PMID: 34685715 Free PMC article.
-
Antifungal Innate Immunity.Adv Exp Med Biol. 2025;1476:225-250. doi: 10.1007/978-3-031-85340-1_9. Adv Exp Med Biol. 2025. PMID: 40622545 Review.
-
Effects of dimerization on the structure and biological activity of antimicrobial peptide Ctx-Ha.Antimicrob Agents Chemother. 2012 Jun;56(6):3004-10. doi: 10.1128/AAC.06262-11. Epub 2012 Mar 5. Antimicrob Agents Chemother. 2012. PMID: 22391524 Free PMC article.
-
Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37.Int J Mol Sci. 2021 Sep 30;22(19):10633. doi: 10.3390/ijms221910633. Int J Mol Sci. 2021. PMID: 34638975 Free PMC article.
-
Resolving the Temporal Splenic Proteome during Fungal Infection for Discovery of Putative Dual Perspective Biomarker Signatures.J Am Soc Mass Spectrom. 2023 Sep 6;34(9):1928-1940. doi: 10.1021/jasms.3c00114. Epub 2023 May 24. J Am Soc Mass Spectrom. 2023. PMID: 37222660 Free PMC article.
References
-
- Odds FC. Candida and candidosis. Maryland: University Park Press; 1979. 382
-
- Calderone RA. Candida and Candidiasis. Washington DC: ASM Press; 2002. 451
-
- Kauffman CA. Fungal infections. Proc Am Thorac Soc. 2006;3:35–40. - PubMed
-
- Wenzel RP. John E. Bennett Forum on Deep Mycoses Study Design 2004: Candidiasis and salvage therapy for aspergillosis. Clin Infect Dis. 2005;41(Suppl 6):S369–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

