Synthesis of cationic dibenzosemibullvalene-based phase-transfer catalysts by di-π-methane rearrangements of pyrrolinium-annelated dibenzobarrelene derivatives
- PMID: 21448242
- PMCID: PMC3063005
- DOI: 10.3762/bjoc.7.17
Synthesis of cationic dibenzosemibullvalene-based phase-transfer catalysts by di-π-methane rearrangements of pyrrolinium-annelated dibenzobarrelene derivatives
Abstract
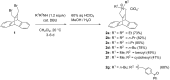
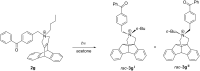
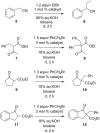
Dibenzobarrelene derivatives, that are annelated with a pyrrolinium unit [N,N-dialkyl-3,4-(9',10'-dihydro-9',10'-anthraceno-3-pyrrolinium) derivatives], undergo a photo-induced di-π-methane rearrangement upon triplet sensitization to give the corresponding cationic dibenzosemibullvalene derivatives [N,N-dialkyl-3,4-{8c,8e-(4b,8b-dihydrodibenzo[a,f]cyclopropa[cd]pentaleno)}pyrrolidinium derivatives]. Whereas the covalent attachment of a benzophenone functionality to the pyrrolinium nitrogen atom did not result in an internal triplet sensitization, the introduction of a benzophenone unit as part of the counter ion enables the di-π-methane rearrangement of the dibenzobarrelene derivative in the solid-state. Preliminary experiments indicate that a cationic pyrrolidinium-annelated dibenzosemibullvalene may act as phase-transfer catalyst in alkylation reactions.
Keywords: di-π-methane rearrangement; dibenzobarrelenes; dibenzosemibullvalenes; phase-transfer catalysis; photochemistry; polycylic compounds.
Figures



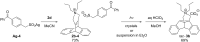
Similar articles
-
Effects of anion complexation on the photoreactivity of bisureido- and bisthioureido-substituted dibenzobarrelene derivatives.Beilstein J Org Chem. 2011 Mar 4;7:278-89. doi: 10.3762/bjoc.7.37. Beilstein J Org Chem. 2011. PMID: 21448249 Free PMC article.
-
The triplet surface of the Zimmerman di-π-methane rearrangement of dibenzobarrelene.Angew Chem Int Ed Engl. 2012 Dec 21;51(52):13097-100. doi: 10.1002/anie.201208002. Epub 2012 Nov 26. Angew Chem Int Ed Engl. 2012. PMID: 23184767
-
Medium-dependent type selectivity in photoreactions of a crown ether-annelated dibenzobarrelene derivative.Org Lett. 2002 Sep 19;4(19):3247-50. doi: 10.1021/ol026482w. Org Lett. 2002. PMID: 12227760
-
Photoalignment layers for liquid crystals from the di-π-methane rearrangement.J Am Chem Soc. 2013 Jan 16;135(2):640-3. doi: 10.1021/ja312090p. Epub 2013 Jan 2. J Am Chem Soc. 2013. PMID: 23281808
-
Recent advances in cinchona alkaloid catalysis for enantioselective carbon-nitrogen bond formation reactions.Curr Top Med Chem. 2014;14(2):224-8. doi: 10.2174/1568026613666131213160228. Curr Top Med Chem. 2014. PMID: 24359199 Review.
References
-
- Armesto D, Ortiz M J, Agarrabeitia A R. Di-π-Methane Rearrangement In Synthetic Organic Photochemistry. In: Griebeck A, Mattay J, editors. Molecular and Supramolecular Photochemistry. Vol. 12. New York: Marcel Dekker; 2005. pp. 161–181.
-
- Ciganek E. J Am Chem Soc. 1966;88:2882–2883. doi: 10.1021/ja00964a068. - DOI
-
- Armesto D. The Aza-di-π-methane Rearrangement. In: Horspool W M, Song P-S, editors. CRC Handbook of Organic Photochemistry and Photobiology. 1st ed. New York: CRC Press; 1995. pp. 915–930. For aza-DPM rearrangements.
-
- Singh V. Photochemical Rearrangements in β,γ-Unsaturated Enones, The Oxa-di-π-methane Rearrangement. In: Horspool W M, Lenci F, editors. CRC Handbook of Organic Photochemistry and Photobiology. 2nd ed. New York: CRC Press; 2004. pp. 78.1–78.34. For oxa-DPM rearrangements.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
