Effects of anion complexation on the photoreactivity of bisureido- and bisthioureido-substituted dibenzobarrelene derivatives
- PMID: 21448249
- PMCID: PMC3063053
- DOI: 10.3762/bjoc.7.37
Effects of anion complexation on the photoreactivity of bisureido- and bisthioureido-substituted dibenzobarrelene derivatives
Abstract
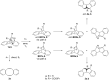
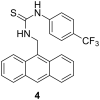
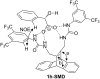
Bisureido- and a bisthioureido-substituted dibenzobarrelene derivative were synthesized and the photoreactivity of two representative examples were studied. Direct irradiation of the ureido-substituted derivative induces a di-π-methane rearrangement to the corresponding dibenzosemibullvalene derivative, whereas the thioureido-substituted derivative is almost photoinert. Complexes of the latter derivative with chloride, carboxylates, or sulfonate anions, however, are efficiently transformed to the dibenzosemibullvalene product upon irradiation, presumably by suppressing the self-quenching of the thiourea units in the complex. The association of the ureido-substituted dibenzobarrelene derivative with (S)-mandelate and irradiation of this complex led to the formation of the dibenzosemibullvalene with moderate stereoselectivity (68:32 er). In contrast, the thioureido derivative showed no such effect upon complexation of chiral anions.
Keywords: (thio)urea derivatives; di-π-methane rearrangement; dibenzobarrelenes; dibenzosemibullvalenes; supramolecular photochemistry.
Figures
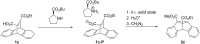

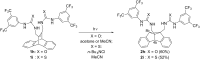

Similar articles
-
Synthesis of cationic dibenzosemibullvalene-based phase-transfer catalysts by di-π-methane rearrangements of pyrrolinium-annelated dibenzobarrelene derivatives.Beilstein J Org Chem. 2011 Jan 26;7:119-26. doi: 10.3762/bjoc.7.17. Beilstein J Org Chem. 2011. PMID: 21448242 Free PMC article.
-
Design of a chiral mesoporous silica and its application as a host for stereoselective di-pi-methane rearrangements.J Org Chem. 2005 Mar 18;70(6):2315-21. doi: 10.1021/jo047878j. J Org Chem. 2005. PMID: 15760220
-
Medium-dependent type selectivity in photoreactions of a crown ether-annelated dibenzobarrelene derivative.Org Lett. 2002 Sep 19;4(19):3247-50. doi: 10.1021/ol026482w. Org Lett. 2002. PMID: 12227760
-
The triplet surface of the Zimmerman di-π-methane rearrangement of dibenzobarrelene.Angew Chem Int Ed Engl. 2012 Dec 21;51(52):13097-100. doi: 10.1002/anie.201208002. Epub 2012 Nov 26. Angew Chem Int Ed Engl. 2012. PMID: 23184767
-
Anion complexation and sensing using modified urea and thiourea-based receptors.Chem Soc Rev. 2010 Oct;39(10):3729-45. doi: 10.1039/b926160p. Epub 2010 Aug 25. Chem Soc Rev. 2010. PMID: 20737072 Review.
Cited by
-
Development of a Highly Enantioselective Catalytic Di-π-methane Rearrangement.J Org Chem. 2024 Dec 6;89(23):17615-17620. doi: 10.1021/acs.joc.4c02383. Epub 2024 Nov 17. J Org Chem. 2024. PMID: 39552035
References
-
- Müller C, Bach T. Aust J Chem. 2008;61:557–564. doi: 10.1071/CH08195. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
