Paclitaxel based vs oxaliplatin based regimens for advanced gastric cancer
- PMID: 21448363
- PMCID: PMC3057154
- DOI: 10.3748/wjg.v17.i8.1082
Paclitaxel based vs oxaliplatin based regimens for advanced gastric cancer
Abstract
Aim: To compare the efficacy and safety of paclitaxel combined with fluorouracil plus cisplatin (PCF), and oxaliplatin combined with fluorouracil plus leucovorin (FOLFOX-4) regimens for advanced gastric cancer (AGC).
Methods: Ninety-four patients with AGC were randomly assigned to receive paclitaxel (50 mg/m(2) iv) on days 1, 8 and 15, cisplatin (20 mg/m(2) iv) and fluorouracil (750 mg/m(2) iv) on days 1-5, or oxaliplatin (85 mg/m(2) iv) and leucovorin (200 mg/m(2) iv) on day 1, followed by bolus fluorouracil (400 mg/m(2) iv) and fluorouracil (600 mg/m(2) iv) on days 1 and 2. The primary end point was the 1-year survival time.
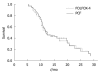
Results: The overall response rate (ORR) of the patients was 48.0% and 45.5% to PCF and FOLFOX-4, respectively. The disease control rate (DCR) of PCF and FOLFOX-4 was 82.0% and 81.8%, respectively. The median survival times (MSTs) of the patients were 10.8 and 9.9 mo, respectively, after treatment with PCF and FOLFOX-4. The 1-year survival rate of the patients was 36.0% and 34.1%, respectively, after treatment with PCF and FOLFOX-4. No significant difference was observed in ORR, DCR, MST or 1-year survival rate between the two groups. The most common adverse events were anemia, nausea and vomiting, and grade 3/4 alopecia in PCF treatment group, and anemia, grade 1/2 neurotoxic effect and grade 3/4 neutropenia in FOLFOX-4 treatment group.
Conclusion: Patients with AGC have a similar response rate to PCF and FOLFOX-4 regimens with a similar survival rate. The PCF and FOLFOX-4 regimens are efficacious and tolerable as a promising therapy for AGC.
Keywords: Advanced gastric cancer; Oxaliplatin; Paclitaxel.
Figures
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Shen X, Zhang J, Yan Y, Yang Y, Fu G, Pu Y. Analysis and estimates of the attributable risk for environmental and genetic risk factors in gastric cancer in a Chinese population. J Toxicol Environ Health A. 2009;72:759–766. - PubMed
-
- Wilke H, Preusser P, Fink U, Achterrath W, Mayer HJ, Stahl M, Lenaz L, Meyer J, Siewert JR, Gerlings H. New developments in the treatment of gastric carcinoma. Cancer Treat Res. 1991;55:363–373. - PubMed
-
- Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315–324. - PubMed
-
- Macdonald JS. Treatment of localized gastric cancer. Semin Oncol. 2004;31:566–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical