Prevalence of adverse drug reactions at a private tertiary care hospital in south India
- PMID: 21448378
- PMCID: PMC3063432
Prevalence of adverse drug reactions at a private tertiary care hospital in south India
Abstract
Background: Adverse Drug Reactions (ADRs) constitute an enormous burden for the society. The aim of the present study was to detect, document, assess and report the suspected ADRs and preparation of guidelines to minimize the incidence of ADRs.
Methods: A prospective-observational study was conducted in the Department of General Medicine of a tertiary care hospital for 12 months from April 2008 to March 2009. Detected and suspected ADRs were analyzed for causality, severity and preventability using appropriate validated scales and were reported. ADR alert card was prepared and given to patients. Therapeutic guidelines were prepared and given to the relevant departments.
Results: A total of 57 ADRs were detected, documented, assessed and reported during the study period the incidence was found to be 1.8%. Assessment of severity of the suspected ADRs revealed that 12% of suspected ADRs were severe and 49% of ADRs were moderate in severity. Causality assessment was done which revealed 63% of ADRs were possibly drug-related. The majority of patients who had suffered from ADRs were above 60 years (56%). Gastrointestinal system was most commonly affected (37%) and the drug class mostly associated with ADRs was antibiotics (23%). Preventability of ADRs was assessed; and the results revealed that 28% of ADRs were definitely preventable.
Conclusions: Measures to improve detection and reporting of adverse drug reactions by all health care professionals is recommended to be undertaken, to ensure, and improve patient's safety. In this way, hospital/clinical pharmacists play the cornerstone role.
Keywords: Adverse Drug Reactions (ADR); India; Prevalence.
Figures




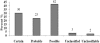
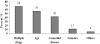
References
-
- Geneva: World Health Organization; 2002. World Health Organization. Safety of medicines - A guide to detecting and reporting adverse drug reactions - Why health professionals need to take actions. Available at: http://apps.who.int/medicinedocs/en/d/Jh2992e/6.html .
-
- Rabbur RSM, Emmerton L. An introduction to adverse drug reaction reporting system in different countries. Int J Pharm Prac. 2005;13(1):91–100.
-
- Rao PGM, Archana B, Jose J. Implementation and results of an adverse drug reaction reporting programme at an Indian teaching hospital. Indian J Pharmacol. 2006;38(4):293–4.
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5. - PubMed
-
- Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacother. 2006;26(6):735–47. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
