Improved elution conditions for native co-immunoprecipitation
- PMID: 21448433
- PMCID: PMC3063181
- DOI: 10.1371/journal.pone.0018218
Improved elution conditions for native co-immunoprecipitation
Abstract
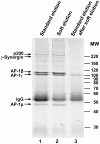
Background: Native immunoprecipitation followed by protein A-mediated recovery of the immuno-complex is a powerful tool to study protein-protein interactions. A limitation of this technique is the concomitant recovery of large amounts of immunoglobulin, which interferes with down-stream applications such as mass spectrometric analysis and Western blotting. Here we report a detergent-based "soft" elution protocol that allows effective recovery of immunoprecipitated antigen and binding partners, yet avoids elution of the bulk of the immunoglobulin.
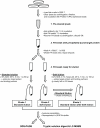
Methodology/principal findings: We assessed the performance of the soft elution protocol using immunoprecipitation of Adaptor protein complex 1 (AP-1) and associated proteins as a test case. Relative to conventional elution conditions, the novel protocol substantially improved the sensitivity of mass spectrometric identification of immunoprecipitated proteins from unfractionated solution digests. Averaging over three independent experiments, Mascot scores of identified AP-1 binding partners were increased by 39%. Conversely, the estimated amount of recovered immunoglobulin was reduced by 44%. We tested the protocol with five further antibodies derived from rabbit, mouse and goat. In each case we observed a significant reduction of co-eluting immunoglobulin.
Conclusions/significance: The soft elution protocol presented here shows superior performance compared to standard elution conditions for subsequent protein identification by mass spectrometry from solution digests. The method was developed for rabbit polyclonal antibodies, but also performed well with the tested goat and mouse antibodies. Hence we expect the soft elution protocol to be widely applicable.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

