Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer
- PMID: 21448449
- PMCID: PMC3063116
- DOI: 10.2147/DDDT.S13029
Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer
Erratum in
- Drug Des Devel Ther. 2011;5:183
Abstract
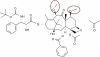
Until recently, patients with castration-resistant prostate cancer (CRPC) had limited therapeutic options once they became refractory to docetaxel chemotherapy, and no treatments improved survival. This changed in June 2010 when the Food and Drug Administration (FDA) approved cabazitaxel as a new option for patients with CRPC whose disease progresses during or after docetaxel treatment. For most of these patients, cabazitaxel will now replace mitoxantrone (a drug that was FDA-approved because of its palliative effects) as the treatment of choice for docetaxel-refractory disease. The approval of cabazitaxel was based primarily on the TROPIC trial, a large (n = 755) randomized Phase III study showing an overall median survival benefit of 2.4 months for men with docetaxel-pretreated metastatic CRPC receiving cabazitaxel (with prednisone) compared to mitoxantrone (with prednisone). Cabazitaxel is a novel tubulin-binding taxane that differs from docetaxel because of its poor affinity for P-glycoprotein (P-gp), an ATP-dependent drug efflux pump. Cancer cells that express P-gp become resistant to taxanes, and the effectiveness of docetaxel can be limited by its high substrate affinity for P-gp. Preclinical and early clinical studies show that cabazitaxel retains activity in docetaxel-resistant tumors, and this was confirmed by the TROPIC study. Common adverse events with cabazitaxel include neutropenia (including febrile neutropenia) and diarrhea, while neuropathy was rarely observed. Thus, the combination of cabazitaxel and prednisone is an important new treatment option for men with docetaxel-refractory metastatic CRPC, but this agent should be administered cautiously and with appropriate monitoring (especially in men at high risk of neutropenic complications).
Keywords: cabazitaxel; castration-resistant prostate cancer; clinical trial; docetaxel resistance; drug development.
Figures
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
-
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. - PubMed
-
- Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170(15):1390–1395. - PubMed
-
- Antonarakis ES, Trock BJ, Feng Z, et al. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up. J Clin Oncol. 2009;27(Suppl) abstract 5008.
-
- D’Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–4573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

