The creatine kinase system and pleiotropic effects of creatine
- PMID: 21448658
- PMCID: PMC3080659
- DOI: 10.1007/s00726-011-0877-3
The creatine kinase system and pleiotropic effects of creatine
Abstract
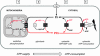
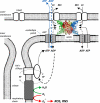
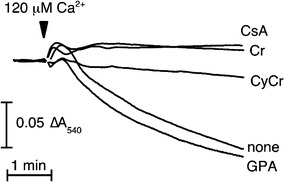
The pleiotropic effects of creatine (Cr) are based mostly on the functions of the enzyme creatine kinase (CK) and its high-energy product phosphocreatine (PCr). Multidisciplinary studies have established molecular, cellular, organ and somatic functions of the CK/PCr system, in particular for cells and tissues with high and intermittent energy fluctuations. These studies include tissue-specific expression and subcellular localization of CK isoforms, high-resolution molecular structures and structure-function relationships, transgenic CK abrogation and reverse genetic approaches. Three energy-related physiological principles emerge, namely that the CK/PCr systems functions as (a) an immediately available temporal energy buffer, (b) a spatial energy buffer or intracellular energy transport system (the CK/PCr energy shuttle or circuit) and (c) a metabolic regulator. The CK/PCr energy shuttle connects sites of ATP production (glycolysis and mitochondrial oxidative phosphorylation) with subcellular sites of ATP utilization (ATPases). Thus, diffusion limitations of ADP and ATP are overcome by PCr/Cr shuttling, as most clearly seen in polar cells such as spermatozoa, retina photoreceptor cells and sensory hair bundles of the inner ear. The CK/PCr system relies on the close exchange of substrates and products between CK isoforms and ATP-generating or -consuming processes. Mitochondrial CK in the mitochondrial outer compartment, for example, is tightly coupled to ATP export via adenine nucleotide transporter or carrier (ANT) and thus ATP-synthesis and respiratory chain activity, releasing PCr into the cytosol. This coupling also reduces formation of reactive oxygen species (ROS) and inhibits mitochondrial permeability transition, an early event in apoptosis. Cr itself may also act as a direct and/or indirect anti-oxidant, while PCr can interact with and protect cellular membranes. Collectively, these factors may well explain the beneficial effects of Cr supplementation. The stimulating effects of Cr for muscle and bone growth and maintenance, and especially in neuroprotection, are now recognized and the first clinical studies are underway. Novel socio-economically relevant applications of Cr supplementation are emerging, e.g. for senior people, intensive care units and dialysis patients, who are notoriously Cr-depleted. Also, Cr will likely be beneficial for the healthy development of premature infants, who after separation from the placenta depend on external Cr. Cr supplementation of pregnant and lactating women, as well as of babies and infants are likely to be of benefit for child development. Last but not least, Cr harbours a global ecological potential as an additive for animal feed, replacing meat- and fish meal for animal (poultry and swine) and fish aqua farming. This may help to alleviate human starvation and at the same time prevent over-fishing of oceans.
Figures

References
-
- Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002;277:24427–24434. - PubMed
-
- Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev Neurosci. 2002;24:382–388. - PubMed
-
- Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG. Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol. 2006;576:391–401. - PMC - PubMed
-
- Aloia JF, Vaswani A, Flaster E, Ma R. Relationship of body water compartments to age, race, and fat-free mass. J Lab Clin Med. 1998;132:483–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

